Molecules
Most of the compounds of interest to biologists are composed of units called molecules.
A molecule is a precise arrangement of atoms held together by chemical bonds, and a compound is a molecule that contains atoms of more than one element.
A molecule may be composed of two or more atoms of the same element, as in oxygen gas (O2), or it may be composed of atoms from different elements.
The arrangements of the atoms in a molecule account for the properties of a compound.
The molecular weight is equal to the atomic weights of the atoms in the molecule.
The atoms in molecules may be joined to one another by various linkages called bonds.
One example of a bond is an ionic bond, which is formed when the electrons of one atom transfer to a second atom.
This creates electrically charged atoms called ions.
The electrical charges cause the ions to be attracted to one another, and the attraction forms the ionic bond.
A second type of linkage is a covalent bond.
A covalent bond forms when two atoms share one or more electrons with one another. For example, as shown in the Figure below, oxygen shares its electrons with two hydrogen atoms, and the resulting molecule is water (H2O).
Nitrogen shares its electrons with three hydrogen atoms, and the resulting molecule is ammonia (NH3).
If one pair of electrons is shared, the bond is a single bond; if two pairs are shared, it is a double bond.
Formation of a covalent bond in water and ammonia molecules.
In each molecule, the second shell fills with eight electrons.
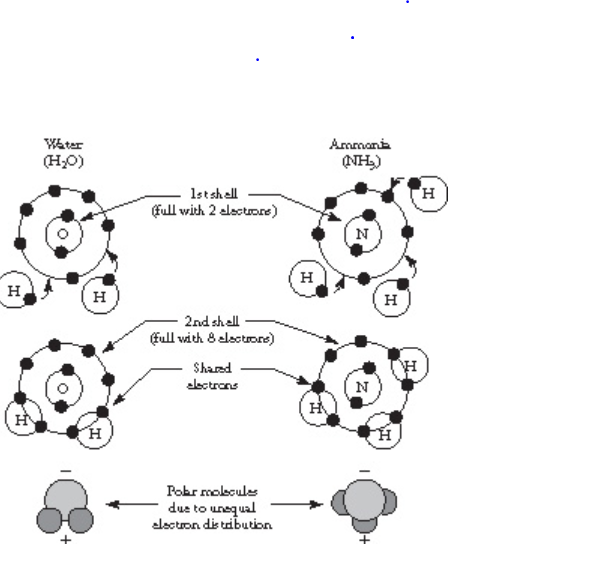
The figure above shows Formation of a covalent bond in water and ammonia molecules.
In each molecule, the second shell fills with eight electrons.
Scholarship 2025/26
Current Scholarships 2025/2026 - Fully FundedFull Undergraduate Scholarships 2025 - 2026
Fully Funded Masters Scholarships 2025 - 26
PhD Scholarships for International Students - Fully Funded!
Funding Opportunities for Journalists 2025/2026
Funding for Entrepreneurs 2025/2026
***
