KCSE Past Papers 2019 Chemistry paper 1(233/1)
Chemistry - Paper 1 - November 2019 - 2 Hours
2019 Chemistry paper 1
1. An atom of element A has mass number 39 and 19 protons.
(a) Write the electron arrangements of the atom (1 mark)
(b) State the period and group to which element A belongs Group ........................................(½ mark)
Period .........................................(½ mark)
(c) State whether the element is a metal or a non-metal. (1 mark)
2. Describe how an increase in concentration increases the rate of a reaction. (2 mark)
3. The flow chart in Figure 1 represents some stages in the extraction of copper metal. Study it and answer the questions that follow.
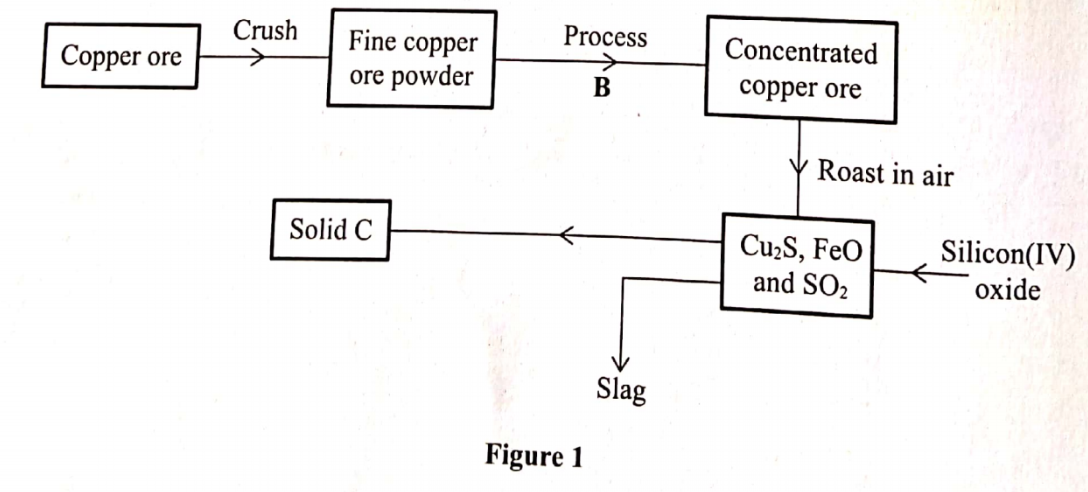
(i) The copper ore (1 mark)
(ii) Process B (½ mark)
(iii) Solid C (½ mark)
(b) Write an equation for the reaction that forms the slag. (1 mark)
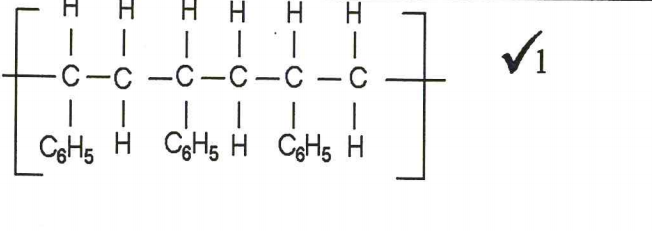
4. A monomer has the following structure.
CH= CH2 ∣ C6H5
(a) Draw the structure of its polymer that contains three monomers.(1 mark)
(b) A sample of the polymer formed from the monomer has a molecular mass of 4992.
Determine the number of monomers that formed the polymer (C= 12; H= 1.0). (2 marks)
5. Hydrogen has can be prepared by passing steam over heated magnesium ribbon as shown in the figure 2.
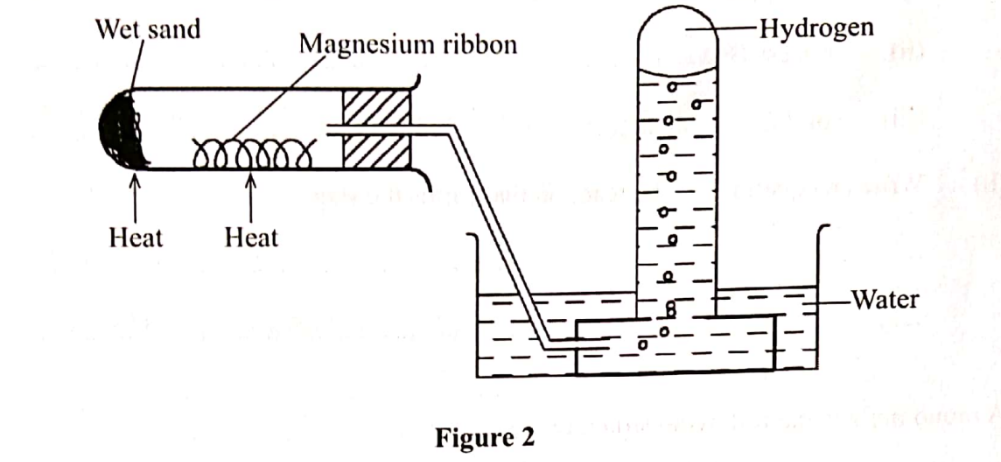
(b) Explain why the delivery tube must be removed from beneath the water before heating is stopped. (1 mark)
(c) Explain why sodium metal is not suitable for this experiment. (I mark)
6. A farmer intended to plant cabbages in his farm. He first tested the pH of the soil and found it to be 3.0.
If cabbages do well in alkaline soils, explain the advice that would be given to the farmer in order to realise a high yield.(2 marks)
7. A solution contains 40.3g of substance XOH per litre .250.0cm3of this solution required 30.0cm3 of 0.3M sulphuric(VI)acid for complete neutralisation. (a) Calculate the number of moles of XOH that reacted. (½ mark)
(b) Determine the relative atomic mass of X. (1½ mark)
8. Table 1 shows the properties of two chlorides, D and E.
Table 1
| Chloride | Melting Points(°C) | Electrical Conductivity (liquid) |
|---|---|---|
| D | 1074 | Good |
| E | 203 | Poor |
(i) D.......................(1 mark)
(ii) E.......................(1 mark)
(b) Explain in terms of structure and bonding, the difference in electrical activity of the chlorides D and E. (1 mark)
9. Sulphur(IV) oxide is prepared in the laboratory using the set-up in Figure 3.
Study it and answer the questions that follow.
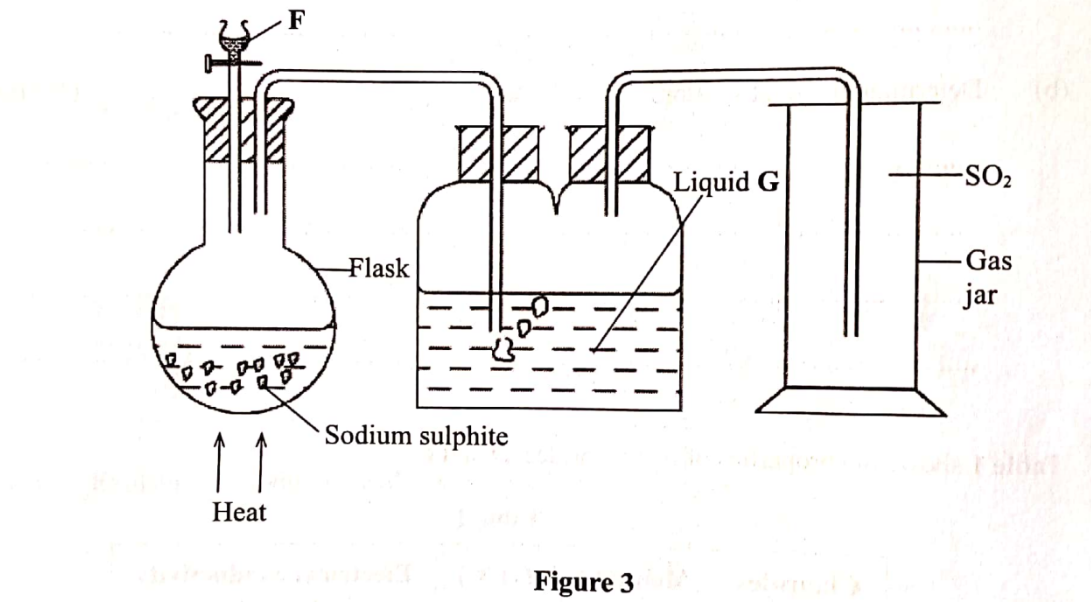
(b) Write an equation for the reaction that takes place in the flask. (1 mark)
(c) State the purpose of liquid G. (1 mark)
The graph in Figure 4 was obtained when a certain substance was heated and its temperature recorded at regular intervals.
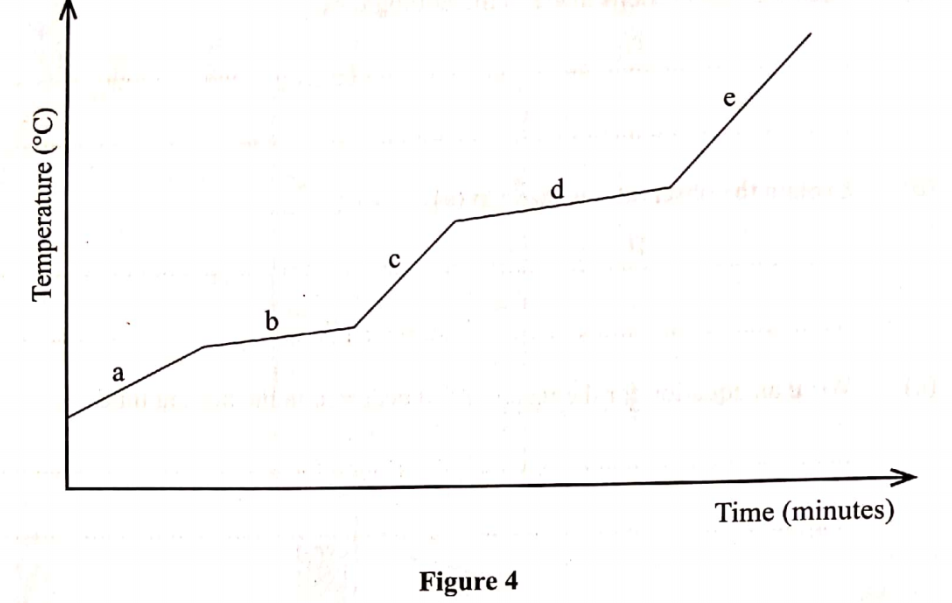
(b) Explain the answer in (a). (1 mark)
11. Ethene is prepared in the laboratory by dehydration of ethanol.
(a) Name a suitable dehydrating agent used in this process. (1 mark)
(b) State the condition necessary for the reaction to occur. (1 mark)
(C) Write an equation for the dehydration process. (1 mark)
112.A boiling yube filled with chlorine was inverted in a trough containing the same ssolution and the set-up left in sunlight for about 2 hours.
(a) State the observation made in the boiling tube ( 1 mark)
(b) Explain the observation made in (a) (1 mark)
(c) Write an equation for the reaction that occurred in the boiling tube (1 mark)
13. 5 g of calcium carbonate was strongly heated to a constant mass.
Calculate the mass of the solid residue formed (Ca = 40.0; C = 12.0; 0 = 16.0). (2 marks)
14. During laboratory preparation of oxygen, manganese(IV) oxide is added to reagent 11.
(a) Name reagent H. (1 mark)
(b) State the role of manganese(IV) oxide in this experiment. (1 mark)
(c) Write the equation for the reaction that takes place. ( I mark)
15. Figure 5 shows an apparatus used to separate a mixture of water and hexene.
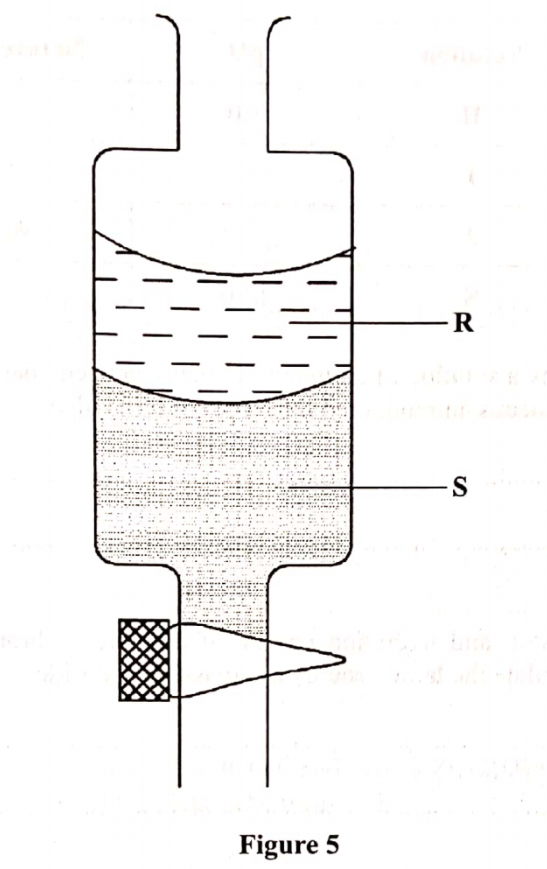
(b) State the principle by which the mixture of the two liquids is separated (1 mark)
(c) Identify the liquids, R and S if the density of hexene is 0.66 g/cm3.
(i) R ............... (½ mark)
(ii) S ...................... (½ mark)
16.(a) Complete the following table.(2 mark)
| Solution | pH | Nature of solution |
|---|---|---|
| H | 1.0 | |
| I | Neutral | |
| J | Weak acid | |
| K | 13.0 |
17. The heat of solution and hydration energy of potassium chloride is — 17.2 kJ and —689 kJ respectively.
Calculate the lattice energy of potassium chloride. (2 marks)
18. Use the information in Table 2 to answer the questions that follow.
| Bond | Bond energy(KJ mol(<sub>-1</sub> |
|---|---|
| C-H | 412 |
| CI-CI | 242 |
| C-CI | 338 |
| H-C1 | 431 |
20. During labomtory preparation of carbon(IV) oxide g tS. substance L in a conical flask.
(a) Identify substance L. (1 mark)
(b) Write an equation that produces carbon(IV) oxide. (1 mark)
(c) State the observations made when the gas produced WHS bubbled through calcium hydroxide solution for a long time. (1 mark)
21. Study the information in Table 3 and use it to answer the questions that follow. (I mark)
| Elements | Na | Mg | Al | Si | P | S | Cl |
|---|---|---|---|---|---|---|---|
| Atomic Numbers | 11 | 12 | 13 | 14 | 15 | 16 | 17 |
| Atomic radii(nm) | 0.157 | 0.136 | 0.125 | 0.117 | 0.110 | 0.104 | 0.099 |
(b) Explain how the chloride of aluminium differs from those of other metals in the period.(2 marks)
2. The diagram in figure 6 shows radiations emitted by a radioactive sample.
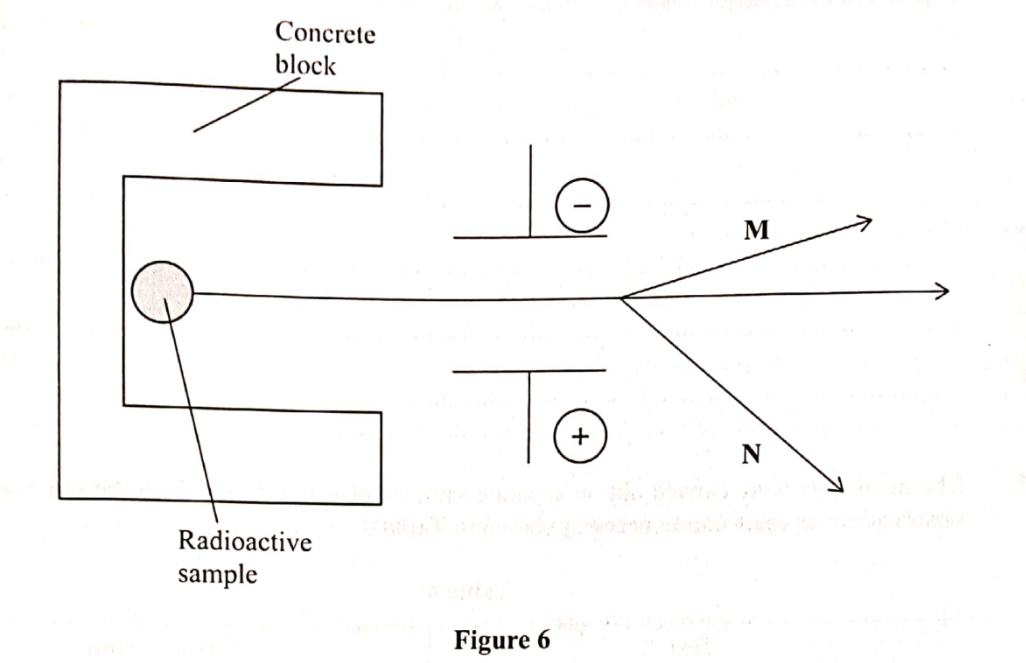
(i) M ................ (1 marks)
(ii) N .................. (1 marks)
(b) Explain what would happen when a sheet of paper is placed in the path of the two radiations. (1 mark)
16/8 X 18/8 X are isotopes of element X.
They occur naturally in the ratio of 9:1 respectively.
Calculate the relative atomic mass of element X. (2 marks)
24. Starting with copper turnings, describe how a sample of copper (II) Sulphate crystals can be prepared in the laboratory. (2 marks)
25. Chemical tests were carried out on separate samples of water drawn from the same source. The observations made were recorded as shown in Table 4.
| Test | Observation |
|---|---|
| (i) Addition of aqueous calcium chloride | No white precipitate |
| (ii) Addition of dilute sulphuric(VI) acid | No effervescence, colourless solution |
| (iii) Addition of a few drops of acidified barium nitrate |
No white precipitate |
| (iv) Addition of aqueous ammonia | White precipitate dissolves |
(i)........................... (1 mark)
(ii)............................. (1 mark)
(iii)..............................(1 mark)
26. 140cm3of nitrogen gas diffuses through a membrane in 70 seconds. How long will it take 200 cm3of carbon(iv) oxide gas to diffuse through the same membrane under the same conditions of temperature and pressure (3 marks)
27. When burning magnesium ribbon is introduced into a gas jar full of nitrogen, it continues to burn producing a greenish yellow powder.
(a) Write an equation for the reaction between nitrogen and magnesium. (I mark)
(b) Explain why magnesium continues to bum in nitrogen but sulphur does not. (2 marks)
(c) State one use of nitrogen. (I mark)
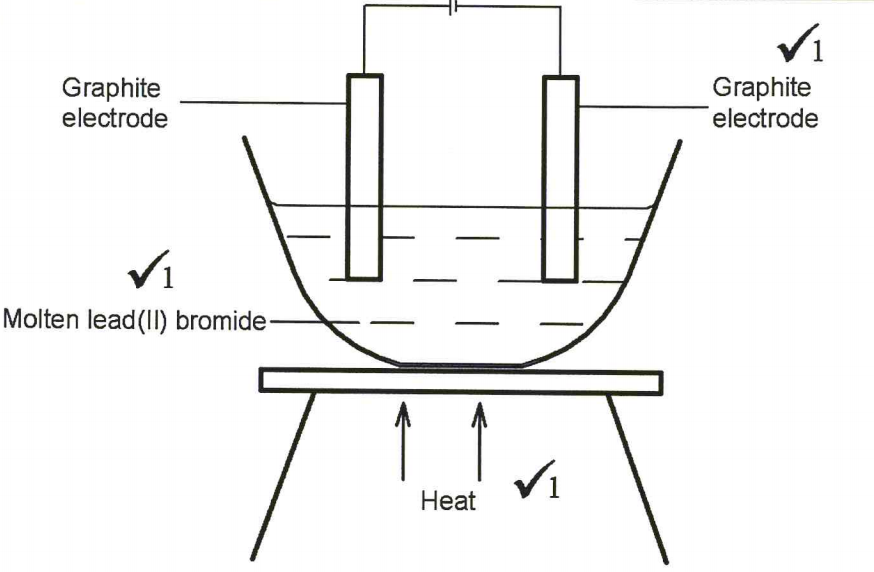
28. Draw in the space provided a labelled diagram of the set-up of the apparatus that can be used to electrolyse molten lead(II) bromide. (3 marks)
29. Name an appropriate apparatus that is used to prepare standard solutions in the laboratory.( I mark)
Questions and Answers
Kenya certificate of Secondary Education
2019 Chemistry paper 1
1. An atom of element A has mass number 39 and 19 protons.
(a) Write the electron arrangements of the atom (1 mark)
(b) State the period and group to which element A belongs Group 1(½ mark)
Period 3(½ mark)
(c) State whether the element is a metal or a non-metal. (1 mark)
2. Describe how an increase in concentration increases the rate of a reaction. (2 mark)
As the concentration increases, the number of reacting particles increases leading to increase in effective collisions. This leads to increase in the rate of reaction.
3. The flow chart in Figure 1 represents some stages in the extraction of copper metal. Study it and answer the questions that follow.

(i) The copper ore (1 mark)
(ii) Process B (½ mark)
(iii) Solid C (½ mark)
(b) Write an equation for the reaction that forms the slag. (1 mark)
FeO(s) + SiO2 (s) —+ FeSiO3(I)
4. A monomer has the following structure.
CH= CH2 ∣ C6H5
(a) Draw the structure of its polymer that contains three monomers.(1 mark)
Determine the number of monomers that formed the polymer (C= 12; H= 1.0). (2 marks)
RFM of monomer = (12 x 8) + 8 =104
Numbers of monomers = 4992/104 = 48units
5. Hydrogen has can be prepared by passing steam over heated magnesium ribbon as shown in the figure 2.

(b) Explain why the delivery tube must be removed from beneath the water before heating is stopped. (1 mark)
(c) Explain why sodium metal is not suitable for this experiment. (I mark)
6. A farmer intended to plant cabbages in his farm. He first tested the pH of the soil and found it to be 3.0.
If cabbages do well in alkaline soils, explain the advice that would be given to the farmer in order to realise a high yield.(2 marks)
7. A solution contains 40.3g of substance XOH per litre .250.0cm3of this solution required 30.0cm3 of 0.3M sulphuric(VI)acid for complete neutralisation.
(a) Calculate the number of moles of XOH that reacted. (½ mark)
H2SO4(g) + 2XOH(aq) —› X SO4(aq) + 2H2 O(l)
Moles of H2SO4 = 30 x 0.3/1000 = 0.009 moles
Moles of XOH 2 x 0.009 0.018 moles
(b) Determine the relative atomic mass of X. (1½ mark)
Molarity of XOH 0.018 x1000/25
= 0.72M
R.F.M = g /1/molarity = 40.3/0.72 = 55.972 =56
0.72 x +16 +1 = 56
x = 56 - 17
x = 39
8. Table 1 shows the properties of two chlorides, D and E.
Table 1
| Chloride | Melting Points(°C) | Electrical Conductivity (liquid) |
|---|---|---|
| D | 1074 | Good |
| E | 203 | Poor |
(b) Explain in terms of structure and bonding, the difference in electrical activity of the chlorides D and E. (1 mark)
9. Sulphur(IV) oxide is prepared in the laboratory using the set-up in Figure 3.
Study it and answer the questions that follow.

(b) Write an equation for the reaction that takes place in the flask. (1 mark)
Na2SO3(s) + 2HC1(Sq) SO z(8) + 2NaCl(dQ) + H2O (1) or Na2SO3(s) +H2SO4 (aq) —+ Na SO4 (aq) + H O(l) +SO2(g)
(c) State the purpose of liquid G. (1 mark)
The graph in Figure 4 was obtained when a certain substance was heated and its temperature recorded at regular intervals.

(b) Explain the answer in (a). (1 mark)
11. Ethene is prepared in the laboratory by dehydration of ethanol.
(a) Name a suitable dehydrating agent used in this process. (1 mark)
(b) State the condition necessary for the reaction to occur. (1 mark)
(C) Write an equation for the dehydration process. (1 mark)
CH3CH2OH H2SO4 CH2CH2 + H2O
12. A boiling yube filled with chlorine was inverted in a trough containing the same solution and the set-up left in sunlight for about 2 hours.
(a) State the observation made in the boiling tube ( 1 mark)
(b) Explain the observation made in (a) (1 mark)
The sunlight decomposed chloric(I) / hypochlorous acid to oxygen and hydrochloric acid.
(c) Write an equation for the reaction that occurred in the boiling tube (1 mark)
2HOCl(aq) —+ 2HCl(aq) + 02 (g)
13. 5 g of calcium carbonate was strongly heated to a constant mass.
Calculate the mass of the solid residue formed (Ca = 40.0; C = 12.0; 0 = 16.0). (2 marks)
CaCO,(s) —› CaO(s) + CO2(g)
No. of moles of CaCO3 = 5/100 = 0.05 moles
100 Moles CaO = 0.05
Mass of CaO = 0.05 x 56
= 2.8g
14. During laboratory preparation of oxygen, manganese(IV) oxide is added to reagent 11.
(a) Name reagent H. (1 mark)
(b) State the role of manganese(IV) oxide in this experiment. (1 mark)
(c) Write the equation for the reaction that takes place. ( I mark)
2H2O2 (1) —+ 2H O(1) + 02 (g)
15. Figure 5 shows an apparatus used to seperate a mixture of water and hexene.

(b) State the principle by which the mixture of the two liquids is separated (1 mark)
(c) Identify the liquids, R and S if the density of hexene is 0.66 g/cm3.
(i) R Hexene (½ mark)
(ii) S Water (½ mark)
16.(a) Complete the following table.(2 mark)
| Solution | pH | Nature of solution |
|---|---|---|
| H | 1.0 | |
| I | Neutral | |
| J | Weak acid | |
| K | 13.0 |
17. The heat of solution and hydration energy of potassium chloride is — 17.2 kJ and —689 kJ respectively.
Calculate the lattice energy of potassium chloride. (2 marks)
Hsoln =H latt + Hhyd
-17.2 = AHlatt + (- 689) AHlatt = +689 -17.2
= +671.8kJmol
18. Use the information in Table 2 to answer the questions that follow.
| Bond | Bond energy(KJ mol(<sub>-1</sub> |
|---|---|
| C-H | 412 |
| CI-CI | 242 |
| C-CI | 338 |
| H-C1 | 431 |
(a) Identify substance L. (1 mark)
Calcium carbonate/CaCO3/Marble chips / ( any other suitable carbonate)
(b) Write an equation that produces carbon(IV) oxide. (1 mark)
CaCO3(S) + 2HCl(aq) —› CaCl2 (aq) + H2O(1) + CO2 (g)
(c) State the observations made when the gas produced WHS bubbled through calcium hydroxide solution for a long time. (1 mark)
21. Study the information in Table 3 and use it to answer the questions that follow. (I mark)
| Elements | Na | Mg | Al | Si | P | S | Cl |
|---|---|---|---|---|---|---|---|
| Atomic Numbers | 11 | 12 | 13 | 14 | 15 | 16 | 17 |
| Atomic radii(nm) | 0.157 | 0.136 | 0.125 | 0.117 | 0.110 | 0.104 | 0.099 |
Atomic radii decreases across the period .Across the period the number of protons increases increasing the nuclear attraction for the outermost electrons contributing to decrease in atomic radii.
(b) Explain how the chloride of aluminium differs from those of other metals in the period.(2 marks)
AlCl3 is molecular/ covalent. It differs from other metal chlorides because it exits as a dimmer. Two molecules of AlCl3 pair through co- ordinate bonds while the other metal chlorides are ionic.
OR AICl3 hydrolyzes in water while the other chlorides do not.
22. The diagram in figure 6 shows radiations emitted by a radioactive sample.

(i) M Alpha α(1 marks)
(ii) N Beta β(1 marks)
(b) Explain what would happen when a sheet of paper is placed in the path of the two radiations. (1 mark)
The alpha (α) particles will be stopped while beta (β) particles will penetrate the sheet of paper. This is because beta particles have higher penetrating power than alpha particles.
23.16/8 X 18/8 X are isotopes of element X.
They occur naturally in the ratio of 9:1 respectively.
Calculate the relative atomic mass of element X. (2 marks)
R.A.M — (9/10x16) +(1/10x18)
— 14.4 +1.8
= 16.2
24. Starting with copper turnings, describe how a sample of copper (II) Sulphate crystals can be prepared in the laboratory. (2 marks)
25. Chemical tests were carried out on separate samples of water drawn from the same source. The observations made were recorded as shown in Table 4.
| Test | Observation |
|---|---|
| (i) Addition of aqueous calcium chloride | No white precipitate |
| (ii) Addition of dilute sulphuric(VI) acid | No effervescence, colourless solution |
| (iii) Addition of a few drops of acidified barium nitrate |
No white precipitate |
| (iv) Addition of aqueous ammonia | White precipitate dissolves |
26. 140cm3of nitrogen gas diffuses through a membrane in 70 seconds. How long will it take 200 cm3of carbon(iv) oxide gas to diffuse through the same membrane under the same conditions of temperature and pressure (3 marks)
(a) Write an equation for the reaction between nitrogen and magnesium. (I mark)
(b) Explain why magnesium continues to bum in nitrogen but sulphur does not. (2 marks)
Burning magnesium produces a lot of heat that is enough to break N-N triple bond hence reacts with it while burning of sulphur produce little heat not enough to break N - N triple bond.
(c) State one use of nitrogen. (I mark)
28. Draw in the space provided a labelled diagram of the set-up of the apparatus that can be used to electrolyse molten lead(II) bromide. (3 marks)
Secondary School Scholarships in Kenya » Kenya Postgraduate Scholarships » Undergraduate Scholarships for Kenyan Students » Kenya Scholarships for Kenyan Students Studying in Kenya » Kenya Undergraduate Scholarships » The Kenya Youth Education Scholarship Fund - Scholarships Kenya - Scholarships KCSE Results » KCSE Results Top 100 Schools - Kenya Certificate of Secondary Education – KCSE » KCSE Top 100 Candidates » Kenya Certificate of Secondary Education – KCSE » KNEC - Kenya National Examinations Council » Secondary Schools in Kenya » KNEC - Kenya National Examinations Council » Free KNEC KCSE Past Papers
Kenya Scholarships for Undergraduate Students » Kenya Scholarships for Postgraduate Students » Undergraduate Scholarships for Kenyan Students » Kenya Undergraduate Scholarships » Full Undergraduate Scholarships for Kenyans » Kenya Postgraduate Scholarships » Scholarships & Grants » Undergraduate Scholarships » Universities in Kenya » Kenya Universities and Colleges Central Placement Service (KUCCPS) » Colleges in Kenya » KASNEB Registration & Results » Secondary Schools Scholarships in Kenya » Undergraduate & Graduate Scholarships for Kenyans
Powerful Motivational Quotes for Students » Success Quotes for Students » KCSE Motivational Quotes for KCSE Candidates » KCSE Success Quotes for KCSE Candidates
1 a a kcse past papers 2014 kcse marking schemes 2016 kcse papers 2016 kcse prediction questions 2018 kcse exam 2018 kcse questions a a kcse past papers advance-africa.com kcse rev quiz agriculture mock papers agriculture paper 2 questions and answers pdf alliance mocks 2017 ap biology essay questions and answers arabic exam 2016 arabic oral exam questions betrayal in the city essay questions and answers pdf betrayal in the city essay questions with answers betrayal in the city, ,,revision questions biology book 3 klb biology essay questions and answers form 4 biology essay questions and answers form 4 pdf biology essays pdf biology exam questions and answers pdf biology form 2 questions and answers pdf biology form 3 notes pdf biology form 3 questions and answers pdf biology form 3 syllabus biology form three reproduction biology form three-questions and answers biology kcse - kcse biology questions and answers - kcse biology essay questions and answers - kcse biology paper 1 2015 - kcse biology notes - kcse 2015 biology paper 2 - kcse biology practical 2015 - kcse biology practicals - kcse biology 2011
biology kcse 2017 biology kcse questions biology paper 1 questions and answers biology paper 2 questions and answers biology paper 3 questions and answers biology questions and answers for high schools biology questions and answers for high schools pdf biology questions and answers form 2 biology questions and answers multiple choice biology questions and answers on cells biology questions and answers online biology questions and answers pdf biology revision notes form 3 business past kcse past papers c.r.e form one notes pdf cambridge igcse computer science cambridge igcse computer science answers cambridge igcse computer science coursebook pdf download cambridge igcse computer science revision guide pdf cambridge igcse computer science study and revision guide pdf cambridge igcse computer science workbook - free download cambridge igcse computer science workbook pdf caucasian chalk circle essay questions chemistry paper 1 questions and answers chemistry paper 2 questions and answers chemistry paper 3 question and answer chemistry past papers form 1 chemistry past papers form 2 cie past papers computer science 0478 computer science igcse past papers xtremepapers computer science paper 2 2017 computer science past papers a level computer science past papers o level computer studies form 1 questions computer studies form 3 past papers computer studies past papers computer studies questions and answers pdf county mocks 2017 cre form 2 notes pdf cre form 3 notes cre form 3 notes pdf cre form 4 notes cre form 4 notes pdf cre form one notes cre kcse 2016 cre notes cre notes form 2 cre notes pdf cre paper 1 with answers cre paper 2 cre paper 2 topics cre preparation notes cre questions form one cre revision notes cre revision questions and answers download kcse past papers with answers dvance kcse past papers edexcel igcse computer science past papers english paper 3 question paper - 2014 kcse english paper 3 question paper - 2015 kcse english paper 3 question paper - 2016 kcse english paper 3 question paper - 2017 kcse english paper 3 question paper - 2018 kcse essay questions and answers on betrayal in the city essay questions based on betrayal in the city find download kcse past papers with answers - kcse past papers pdf download - kcse 2013 marking scheme - kcse mathematics past papers pdf - free kcse past papers and marking schemes - kcse mock papers pdf - kcse past papers 2014 pdf - kcse past papers 2015 - kcse past papers 2010 find kcse biology essay questions and answers - kcse biology practicals - kcse biology paper 1 2015 - biology essay questions and answers form 4 - kcse biology questions and answers - ap biology essay questions and answers - kcse biology notes - kcse biology paper 2 2012 - kcse biology paper 2 2015
form 2 biology questions and answers free kcse mocks 2015 free kcse past papers - kcse past papers - knec kcse online past papers - knec kcse results past papers free kcse past papers 2014 free kcse past papers kenya, free marking schemes, download ... free kcse past papers with answers free kcse questions and answers on chemistry free revision papers general biology test questions and answers general science questions and answers pdf history and government paper one topics history form one questions and answers pdf history paper 1 questions and answers history paper 2 questions and answers home science past papers igcse computer science book igcse computer science book pdf download igcse computer science notes igcse computer science paper 2 notes igcse computer science past papers igcse computer science past papers 2014 igcse computer science past papers 2017 igcse computer science pdf igcse computer science pre release material 2018 igcse computer science resources igcse computer science revision notes pdf igcse computer science workbook pdf igcse computer studies past papers interesting biology questions ire kcse past papers k.c.s.e cre paper 1 2017 k.c.s.e geography 2017 k.c.s.e mathematics paper 1 2017 k.c.s.e mocks 2018 k.c.s.e past papers 2014 kcpe 2018 predictions kcpe prediction questions kcse 2010 marking scheme kcse 2010 past papers kcse 2011 cre paper 1 kcse 2011 marking scheme kcse 2012 history paper 2 marking scheme kcse 2012 marking schemes kcse 2013 cre paper 1 kcse 2013 marking scheme kcse 2013 marking scheme pdf kcse 2014 kcse 2015 biology paper 2 kcse 2015 biology paper 3 kcse 2015 marking scheme kcse 2015 past papers kcse 2016 agriculture paper 2 kcse 2016 biology paper 1 kcse 2016 biology paper 2 kcse 2016 computer paper 1 kcse 2017 marking scheme kcse 2017 maths paper 1 kcse 2017 papers kcse 2017 papers and marking scheme kcse 2017 past papers kcse 2017 prediction pdf kcse 2018 cre prediction kcse 2018 leakage kcse 2018 marking scheme kcse 2018 papers kcse 2018 predictions kcse 2019 marking scheme kcse agriculture past papers kcse answers kcse arabic paper 1 kcse arabic paper 2 kcse arabic paper 3 kcse arabic paper 3 2016 kcse arabic past papers kcse biology 2011 kcse biology essay questions and answers kcse biology essay questions and answers - kcse revision questions and answers - kcse chemistry questions and answers - kcse revision papers with answers - kcse past papers with answers - download kcse past papers with answers - kcse questions on the river and the source - kcse revision notes
kcse biology essay questions and answers - kcse revision questions and answers - kcse chemistry questions and answers - kcse revision papers with answers - kcse past papers with answers - download kcse past papers with answers - kcse questions on the river and the source - kcse revision notes
kcse biology essay questions and answers pdf kcse biology essays kcse biology essays pdf kcse biology notes kcse biology paper 1 kcse biology paper 1 2017 kcse biology paper 1 2017 pdf kcse biology paper 2 2012 kcse biology paper 2 2015 kcse biology paper 2 2017 kcse biology paper 3 2016 kcse biology paper 3 past papers kcse biology past papers kcse biology past papers and answers kcse biology practical 2016 kcse biology practical past papers kcse biology practicals kcse biology questions and answers kcse biology questions and answers - kcse past papers biology - kcse biology essay questions and answers - kcse chemistry past papers - download kcse past papers with answers - k.c.s.e papers 2015 - k.c.s.e papers 2016 - kcse biology paper 1 2015 - kcse past papers 2015 - kcse past papers 2011 - kcse past papers 2016 - kcse past papers 2017 - 2017 kcse prediction questions - 2018 kcse prediction questions
kcse business paper 1 2016 kcse business past papers kcse business studies past papers kcse chemistry paper 1 2016 kcse chemistry paper 1 2017 kcse chemistry paper 3 2012 kcse chemistry past papers kcse chemistry past papers and answers kcse chemistry practical kcse computer studies paper 1 kcse computer studies paper 2 kcse computer studies paper 2 pdf kcse cre 2016 kcse cre paper 1 2013 kcse cre paper 1 2015 kcse cre paper 1 2016 kcse cre paper 1 2017 kcse cre paper 2 kcse cre paper 2 2016 kcse cre past papers kcse cre past papers and answers kcse english paper 3 2016 kcse english paper 3 2017 kcse essay questions in betrayal in the city kcse exam papers 2018 kcse exam papers answers kcse french paper 1 kcse french paper 2 kcse french past papers kcse general science syllabus kcse geography paper 2 2016 kcse history paper 1 2012 kcse history paper 2 2016 kcse history paper 2 2017 kcse kiswahili paper 1 2017 kcse marking scheme 2016 kcse marking schemes kcse marking schemes 2017 kcse marking schemes pdf kcse mathematics marking schemes kcse mathematics paper 1 2015 kcse mathematics paper 1 2016 kcse mathematics paper 2 2016 kcse mathematics past papers kcse mathematics past papers pdf kcse mock exams kcse mock papers 2015 kcse mock papers 2017 kcse mock papers 2018 kcse mock papers pdf kcse mock papers pdf 2018 kcse mocks 2017 kcse mocks 2018 kcse music past papers kcse online past papers kcse papers 2015 kcse past papers kcse past papers - kcpe and answers - free mocks online - kcse answers past exams question papers - downloads | kcse papers and marking schemes | exams - kcse mathematics paper 1 questions and answers - kcse cre paper 1 questions and answers - knec past papers free downloads - kcse online registration - kcpe - kcse past papers - knec - knec portal - knec past papers for colleges - kasneb - past papers - kasneb past papers for colleges - cpa past papers - https://www.knec.ac.ke/ - www.knec-portal.ac.ke/ - knec portal: kcse results, online registration, kcse result slip. knec portal confirmation - knec portal kcse results - knec examiners portal - knec website kcse past papers - kcpe and answers - free mocks online - kcse answers past exams question papers - downloads | kcse papers and marking schemes | exams - kcse mathematics paper 1 questions and answers - kcse cre paper 1 questions and answers
kcse past papers 2007 kcse past papers 2009 kcse past papers 2010 kcse past papers 2011 kcse past papers 2011 pdf kcse past papers 2012 kcse past papers 2013 kcse past papers 2013 -knec kcse past papers 2014 kcse past papers 2014 pdf kcse past papers 2015 kcse past papers 2015 marking schemes kcse past papers 2015 pdf kcse past papers 2016 kcse past papers 2016 pdf kcse past papers 2017 kcse past papers 2017 pdf kcse past papers agriculture and answers kcse past papers arabic and answers kcse past papers art and design and answers kcse past papers biology kcse past papers building and construction and answers kcse past papers business studies and answers kcse past papers chemistry kcse past papers chemistry and answers kcse past papers chemistry pdf kcse past papers computer studies and answers kcse past papers cre and answers kcse past papers electricity and answers kcse past papers english and answers kcse past papers french and answers kcse past papers general science and answers kcse past papers geography and answers kcse past papers german and answers kcse past papers history and government and answers kcse past papers home science and answers kcse past papers hre and answers kcse past papers ire and answers kcse past papers kenya sign language and answers kcse past papers kiswahili and answers kcse past papers marking scheme kcse past papers maths kcse past papers metal work and answers kcse past papers music and answers kcse past papers pdf download kcse past papers physics and answers kcse past papers physics with answers kcse past papers power mechanics and answers kcse past papers with answers kcse past papers woodwork and answers kcse physics past papers kcse prediction 2017 kcse prediction 2018 kcse prediction 2018 pdf kcse prediction papers 2018 kcse prediction questions 2018 kcse prediction questions and answers kcse questions and answers kcse questions and answers. download free kcse past papers from knec. all marking schemes - questions and answers are sourced from knec. kcse revision kcse revision papers 2014 kcse revision | secondary school | text books | text book centre kcse trial 2017 kcse trial exams 2017 kenyaplex kcse past papers kenyaplex past papers for secondary kiswahili paper 3 questions and answers klb biology form 3 pdf klb cre form 1 klb cre form 3 knec ict past papers knec past papers for colleges knec past papers free download knec past papers pdf knec revision papers knec technical exams past papers kusoma.com past papers maths kcse 2017 mock past papers 2017 mock past papers with answers mokasa mock 2017 page navigation papacambridge computer science igcse past kcse papers past papers in kenya pre mocks 2018 pte knec past papers revision sample essays on betrayal in the city school biology notes school geography notes school physics notes school river and the source themes used in betrayal in the city xtremepapers igcse computer science z notes computer science igcse
Scholarship 2024/25
Current Scholarships 2024/2025 - Fully Funded
Full Undergraduate Scholarships 2024 - 2025
Fully Funded Masters Scholarships 2024 - 25
PhD Scholarships for International Students - Fully Funded!
Funding Opportunities for Journalists 2024/2025
Funding for Entrepreneurs 2024/2025
***
