Chemistry Notes - Acid, Bases and Salts
Click Here - Free KCSE Past Papers » KNEC Past Exams » Free Downloads » KCSE Papers & Marking Schemes
A. Acids And Bases
At a school laboratory:
(i)An acid may be defined as a substance that turn litmus red.
(ii)A base may be defined as a substance that turn litmus blue.
Litmus is a lichen found mainly in West Africa. It changes its colour depending on whether the solution it is in, is basic/alkaline or acidic.It is thus able to identify/ show whether
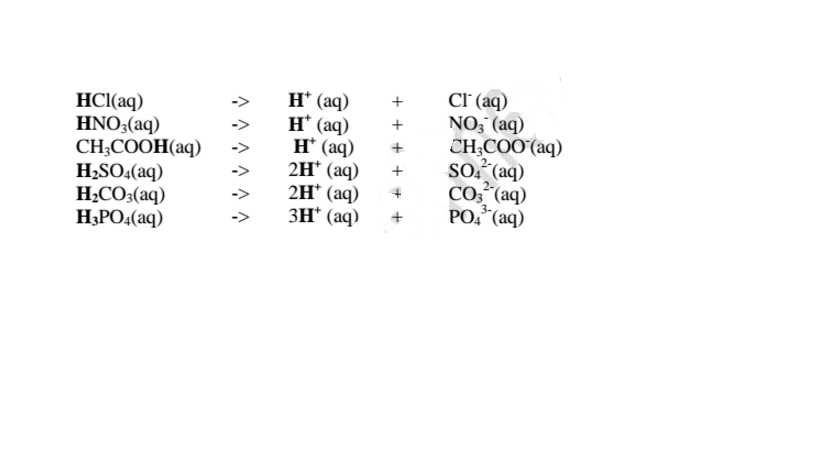
1. An acid is a substance that dissolves in water to form H+/H3O+ as the only positive ion/cation. This is called the Arrhenius definition of an acid. From this definition, an acid dissociate/ionize in water releasing H+ thus:
This is called Arrhenius definition of a base.
From this definition, a base dissociate/ionize in Water releasing OH' thus:

3. An acid is a proton donor.
A base is a proton acceptor.
This is called Bronsted-Lowry definition of acids and bases.
From this definition, an acid donates H+ .
H+ has no electrons and neutrons .It contains only a proton.
Examples
i. From the equation:
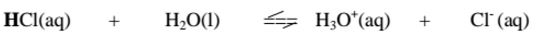
(a)(i)For the forward reaction from left to right, H2O gains a proton to form H3O+ and thus H2o is a proton acceptor.It is a Bronsted-Lowry base
(ii) For the backward reaction from right to left, H3O+ donates a proton to form H2o and thus H3O+ is an ,,opposite" proton donor. It is a Bronsted- Lowry conjugate acid
(b)(i)For the forward reaction from left to right, HCl donates a proton to form Cl‘ and thus HCl is a proton donor.
It is a Bronsted-Lowry acid
(ii) For the backward reaction from right to left, Cl" gains a proton to form HCl and thus Cl' is an ,,opposite" proton acceptor.
It is a Bronsted-Lowry conjugate base.
Every base /acid from Bronsted-Lowry definition thus must have a conjugate product/reactant.
II. From the equation:
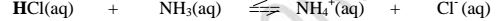
(a)(i)For the forward reaction from left to right, NH; gains a proton to form NH4 and thus NH3; is a proton acceptor .
It is a Bronsted-Lowry base
(ii) For the backward reaction from right to left, NH4+ donates a proton to form NH; and thus NH4+ is an ,,opposite" proton donor.
It is a Bronsted-Lowry conjugate acid
(b)(i)F or the forward reaction from left to right, HCl donates a proton to form C1‘ and thus HCl is a proton donor .
It is a Bronsted-Lowry acid
(ii) For the backward reaction from right to left, Cl" gains a proton to form HCl and thus Cl' is an ,,opposite" proton acceptor.
It is a Bronsted-Lowry conjugate base.
4. Acids and bases show acidic and alkaline properties/characteristics only in Water but not in other solvents e.g.
(a)Hydrogen chloride gas dissolves in water to form hydrochloric acid
Hydrochloric acid dissociates/ionizes in water to free
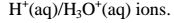
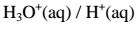
(i)turning blue litmus paper/solution red.
(ii)show pH value 1/2/3/4/5/6
(iii)are good electrolytes/conductors of electricity/undergo electrolysis.
(iv)react with metals to produce /evolve hydrogen gas and a salt. i.e.
Ionically:
lonically:
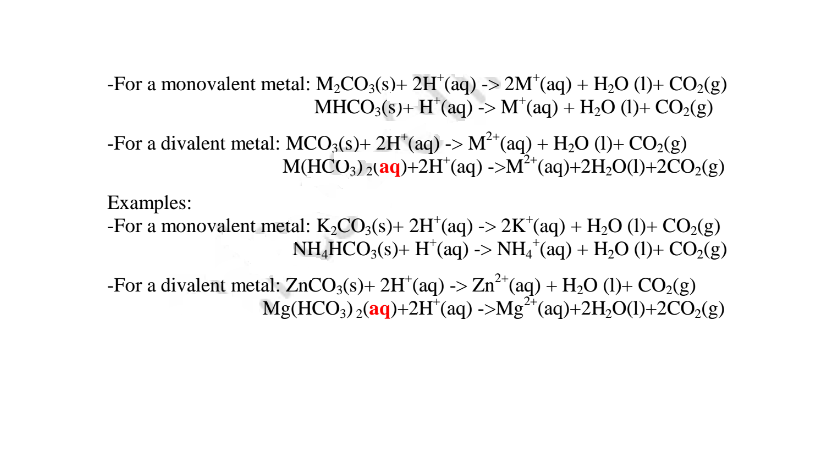
(vi)neutralize metal oxides/hydroxides to salt and water only. i.e. Ionically:
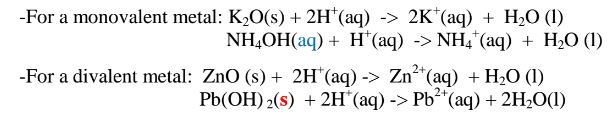
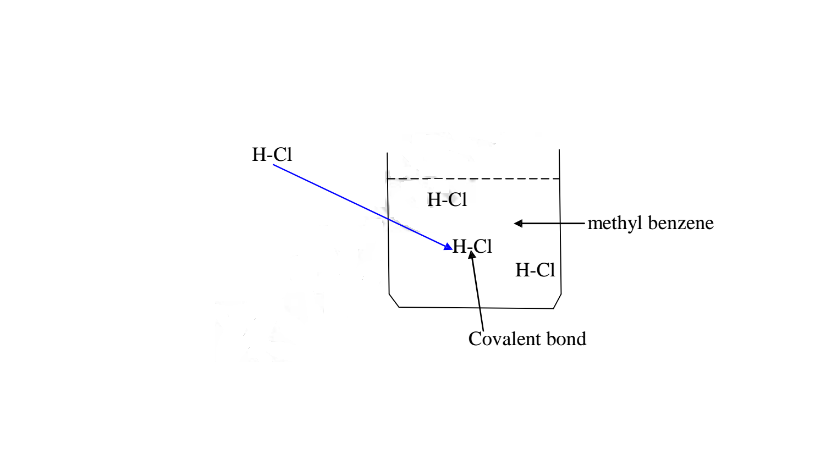
(b)Hydrogen chloride gas dissolves in methylbenzene /benzene but does not dissociate /ionize into free ions.
It exists in molecular state showing none of the above properties.
(c)Ammonia gas dissolves in water to form aqueous ammonia which dissociate/ionize to free NH4+ (aq) and OH'(aq) ions.
This dissociation/ionization makes aqueous ammonia to:
(i)tum litmus paper/solution blue.
(ii)have pH 8/9/ 10/ 11
(iii)be a good electrical conductor
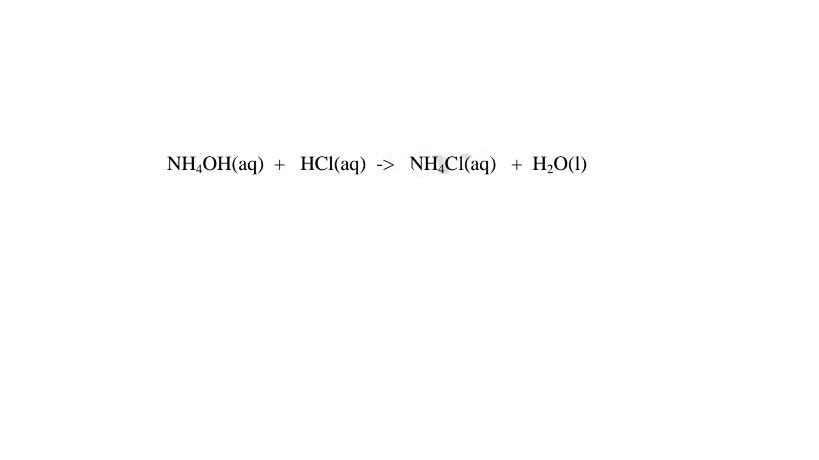
(iv)react with acids to form ammonium salt and water only.
6. Solvents are either polar or non-polar.
A polar solvent is one which dissolves ionic compounds and other polar solvents.
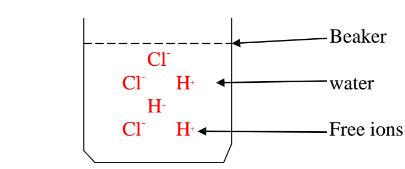
Water is polar solvent that dissolves ionic and polar substance by surrounding the free ions as below:
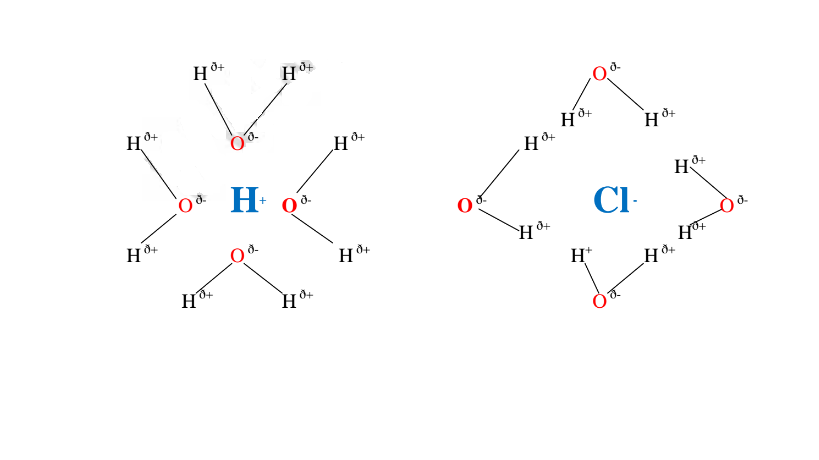
Note:Water is polar .It is made up of :
Oxygen atom is partially negative and two hydrogen atoms which are partially positive.
They surround the free Hl and Cl" ions.
A non polar solvent is one which dissolved non-polar substances and covalent
compounds.
If a polar ionic compound is dissolved in non-polar solvent ,it does not ionize/dissociate into free ions as below:
7. Some acids and bases are strong while others are Weak.
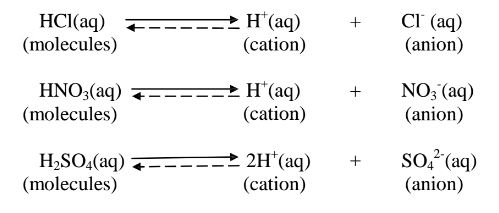
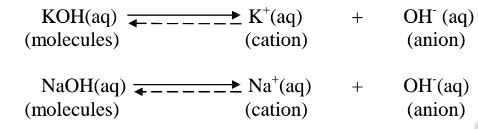
(a)A strong acid/base is one which is fully/wholly/completely dissociated / ionized into many free H+ /OH' ions i.e.
i. Strong acids exists more as free H+ ions than molecules. e. g.
ii. Strong bases/alkalis exists more as free OH' ions than molecules. e.g.
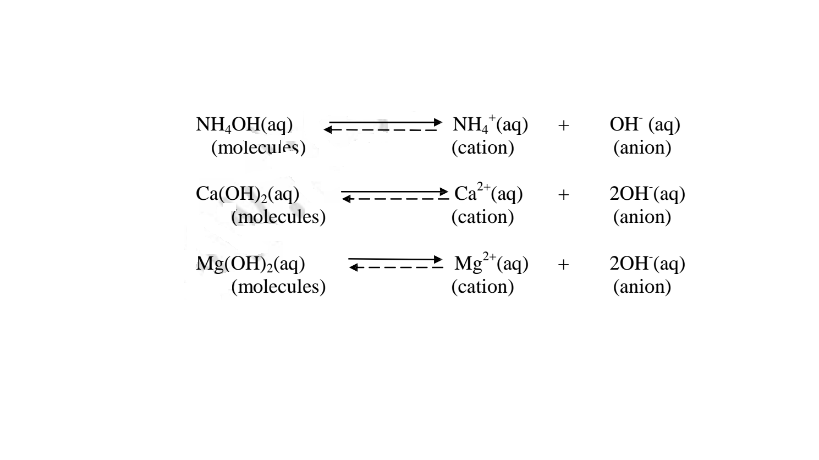
(b) A weak base/acid is one which is paitially /partly dissociated /ionized in water into free OH' (aq) and H+(aq) ions.
i. Weak acids exists more as molecules than as free Hl ions. e.g.
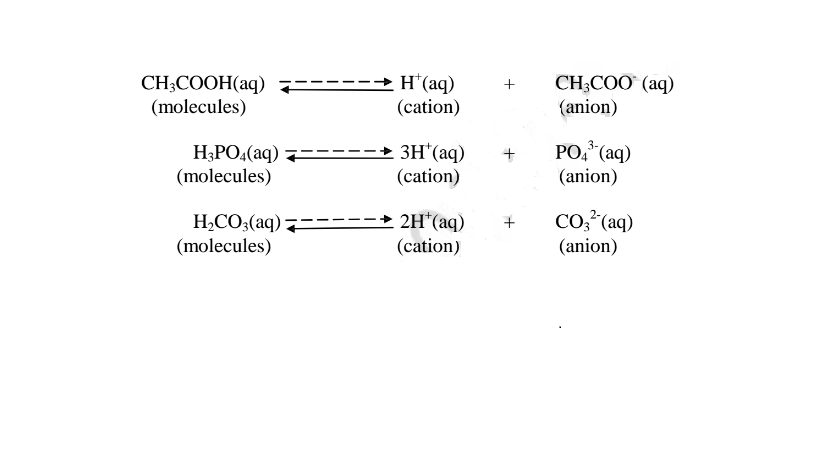
ii. Weak bases/alkalis exists more as molecules than free OH' ions. e. g.
8. The concentration of an acid/base/alkali is based on the number of moles of acid/bases dissolved in a decimeter(litre)of the solution.
An acid/base/alkali With more acid/base/alkali in a decimeter(litre) of solution is said to be concentrated while that with less is said to be dilute.
9. (a) (i)strong acids have pH 1/2/3 while weak acids have high pH 4/5/6.
(ii)a neutral solution have pH 7
(iii)strong alkalis/bases have pH 12/13/14 while weak bases/alkalis have pH 11/10 /9 / 8.
(b) pH is a measure of H+(aq) concentration in a solution.
The higher the H+(aq)ions concentration ;
-the higher the acidity
-the lower the pH
-the lower the concentration of OH_(aq)
-the lower the alkalinity
At pH 7 , a solution has equal concentration of H+(aq) and OH'(aq).
Beyond pH 7,the concentration of the OH'(aq) increases as the H+(aq) ions decreases.
10.(a) When acids /bases dissolve in water, the ions present in the solution conduct electricity.
The more the dissociation the higher the yield of ions and the greater the electrical conductivity of the solution.
A compound that conducts electricity in an electrolyte and thus a compound showing high electrical conductivity is a strong electrolyte while a compound showing low electrical conductivity is a weak electrolyte.
(b) Practically, a bright light on a bulb ,a high voltage reading from a voltmeter high ammeter reading from an ammeter, a big deflection on a galvanometer is an indicator of strong electrolyte(acid/base) and the opposite for weak electrolytes(acids/base)
11. Some compounds exhibit/show both properties of acids and bases/alkalis.
A substance that reacts with both acids and bases is said to be amphotellic.
The examples below show the amphotellic properties of:
(a)
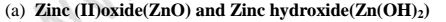
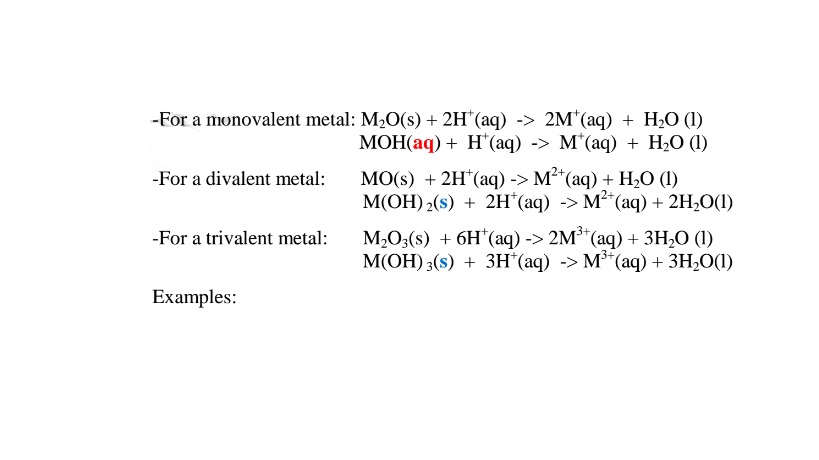
(i)When 1/2 spatula full of Zinc(II)oxide is placed in a boiling tube containing 10cm3 of either 2M nitric(V)acid or 2M sodium hydroxide hydroxide solution, it dissolves on both the acid and the alkali/base to form a colourless solution. i.e.
(i) when reacting with nitric(V)acid, the oxide shows basic properties by reacting with an acid to form a simple salt and water only.
Basic oxide + Acid -> salt + water
Examples:
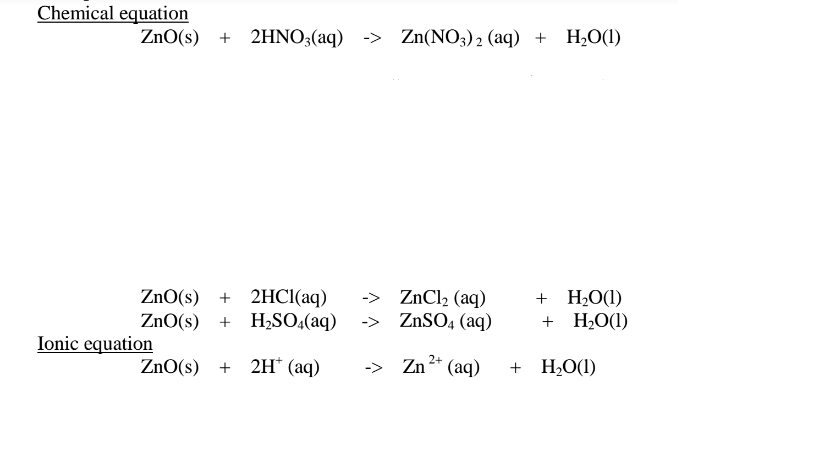
(ii) when reacting with sodium hydroxide, the oxide shows acidic properties by reacting with a base to form a complex salt.
Basic oxide + Base/alkali + Water -> Complex salt
Examples:
Chemical equation
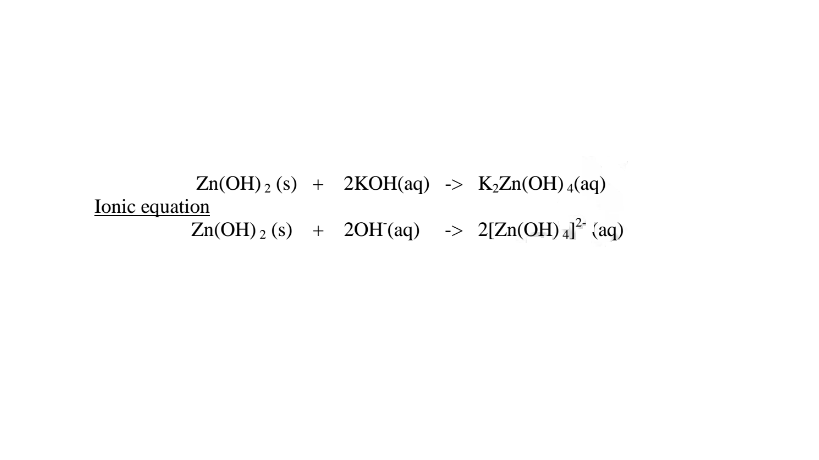
1.When Zinc oxide is reacted with sodium hydroxide the complex salt is sodium tetrahydroxozincate(II) complex salt.
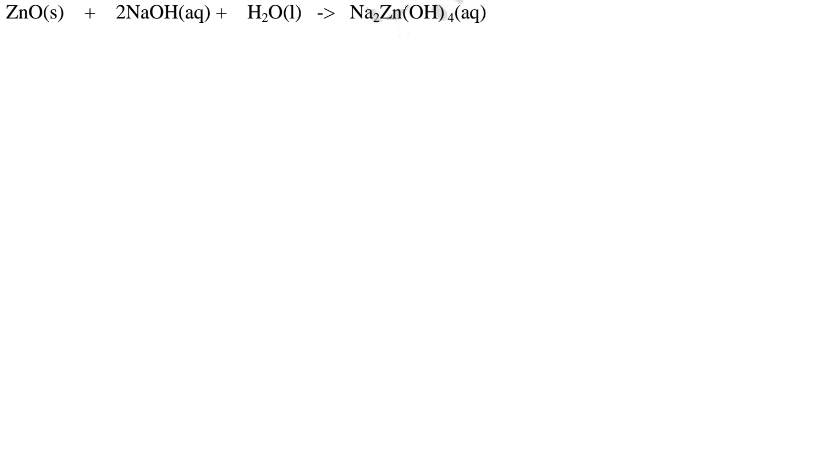
2.When Zinc oxide is reacted with potassium hydroxide the complex salt is potassium tetrahydroxozincate(II) complex salt.
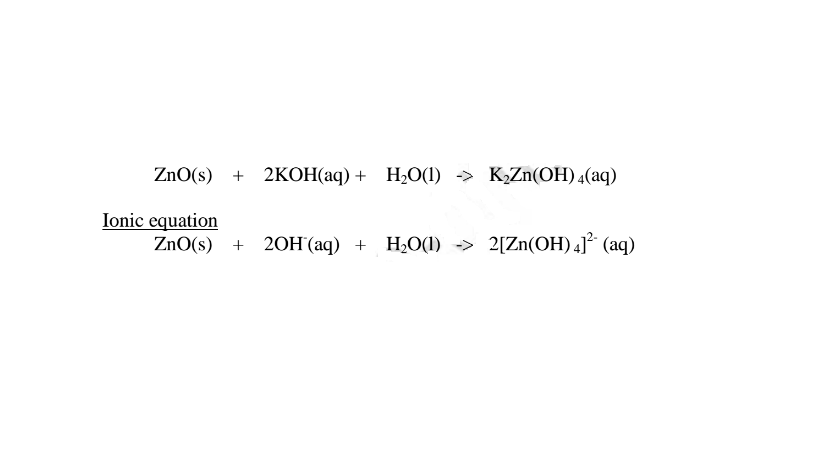
(i) when reacting with nitric(V)acid, the hydroxide shows basic properties. It reacts with an acid to form a simple salt and water only.
Basic hydroxide + Acid -> salt + water
Examples:

Basic hydroxide + Base/alkali -> Complex salt
Examples:
Chemical equation
1.When Zinc hydroxide is reacted with sodium hydroxide the complex salt is sodium tetrahydroxozineate(II) complex salt.
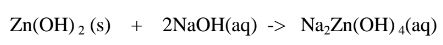
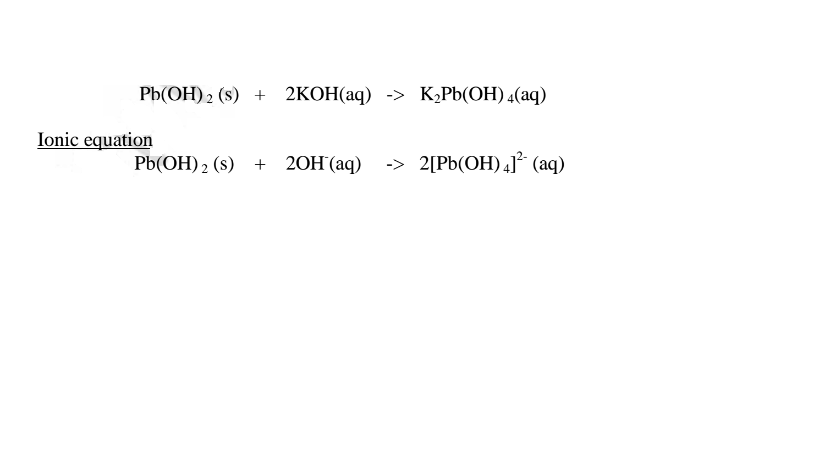
(b) Lead (II)oxide(PbO) and Lead(II) hydroxide (Pb(OH)2)
(i)When 1/2 spatula full of Lead(II)oxide is placed in a boiling tube containing 10cm3 of either 2M nitric(V)acid or 2M sodium hydroxide hydroxide solution, it dissolves on both the acid and the alkali/base to form a colourless solution. i.e.
(i) when reacting with nitric(V)acid, the oxide shows basic properties by reacting with an acid to form a simple salt and water only. All other Lead salts are insoluble.
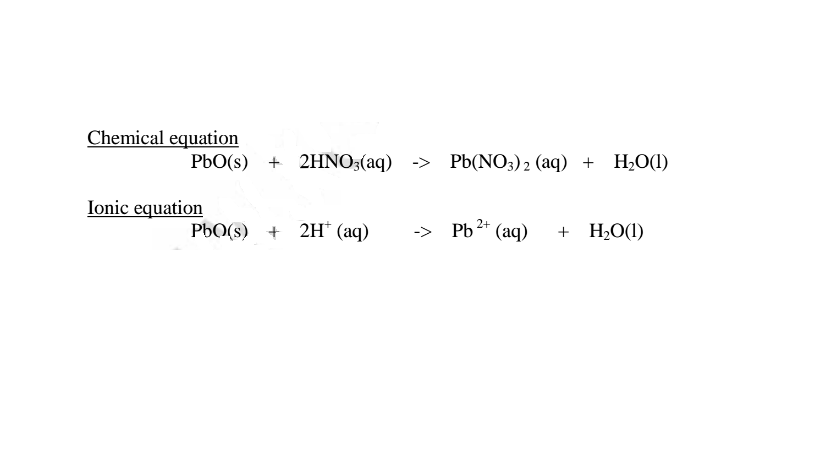
(ii) when reacting with sodium hydroxide, the oxide shows acidic properties by reacting with a base to form a complex salt.
Chemical equation
1.When Lead(II) oxide is reacted with sodium hydroxide the complex salt is sodium tetrahydroxoplumbate(II) complex salt.
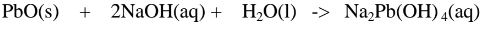
2.When Lead(II) oxide is reacted with potassium hydroxide the complex salt is potassium tetrahydroxoplumbate(II) complex salt.
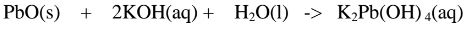
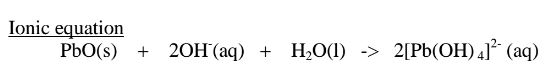
(ii)When Lead(II)hydroxide is placed in a boiling tube containing 10cm3 of either 2M nitric(V)acid or 2M sodium hydroxide hydroxide solution, it dissolves on both the acid and the alkali/base to form a colourless solution. i.e.
(i) when reacting with nitric(V)acid, the hydroxide shows basic properties. It reacts with the acid to form a simple salt and water only.

(ii) when reacting with sodium hydroxide, the hydroxide shows acidic properties. It reacts with a base to form a complex salt.
Chemical equation
1.When Lead(II) hydroxide is reacted with sodium hydroxide the complex salt is sodium tetrahydroxoplumbate(II) complex salt.
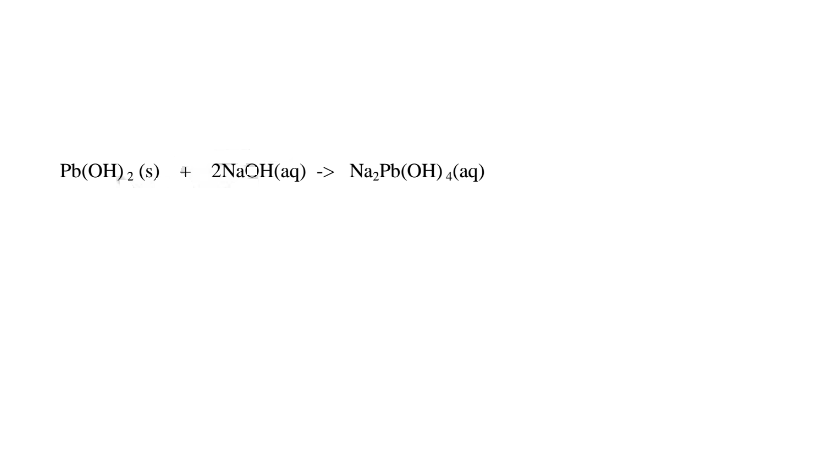
2.When Lead(II) hydroxide is reacted with potassium hydroxide the complex salt is potassium tetrahydroxoplumbate(II) complex salt.
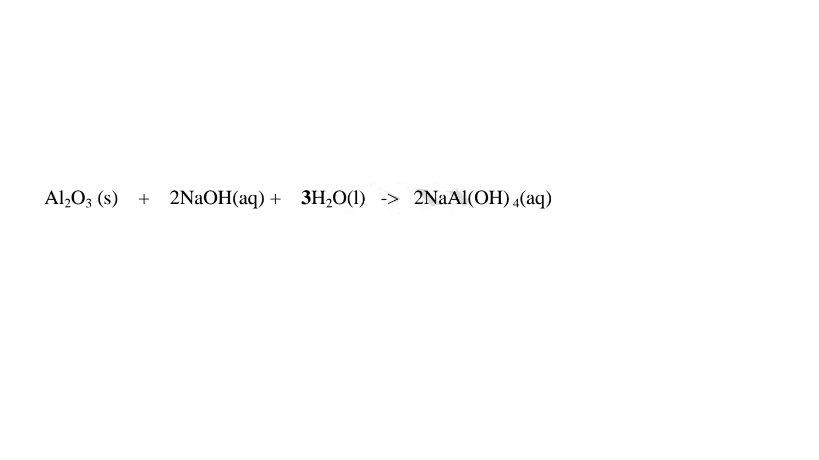
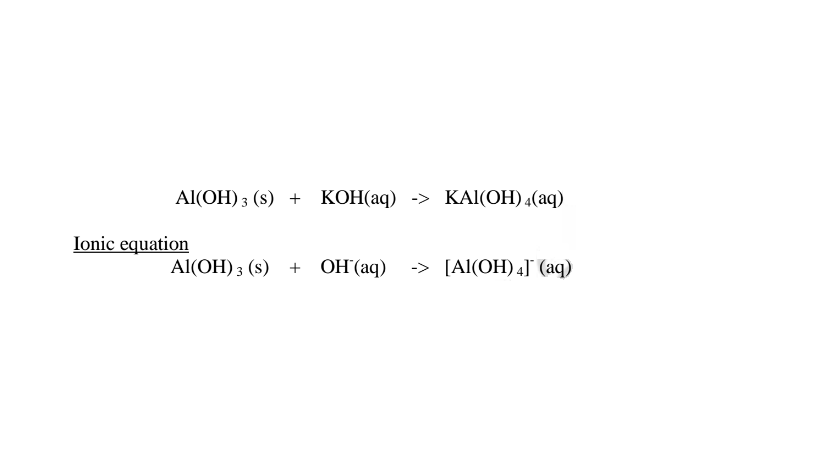
(c)Aluminium(III)0xide(Al2O3) and Aluminium(III)hydroxide(Al(OH)3)
(i)When 1/2 spatula full of Aluminium(III)oxide is placed in a boiling tube containing 10cm3 of either 2M nitric(V)acid or 2M sodium hydroxide hydroxide solution, it dissolves on both the acid and the alkali/base to form a colourless solution. i.e.
(i) when reacting with nitric(V)acid, the oxide shows basic properties by reacting with an acid to form a simple salt and Water only.

Chemical equation
1.When Aluminium(Ill) oxide is reacted with sodium hydroxide the complex salt is sodium tetrahydroxoaluminate(III) complex salt.
2.When Aluminium(III) oxide is reacted with potassium hydroxide the complex salt is potassium tetrahydroxoaluminate(II) complex salt.
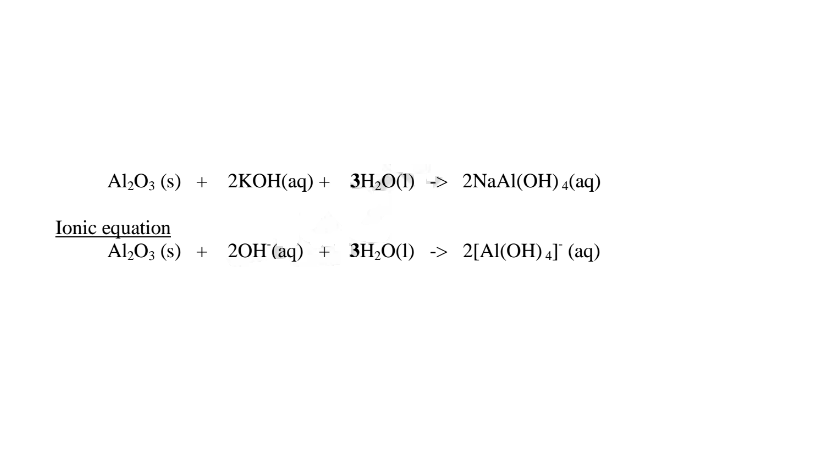
(ii)When Aluminium(IlI)hydroxide is placed in a boiling tube containing 10cm3 of either 2M nitric(V)acid or 2M sodium hydroxide hydroxide solution, it dissolves on both the acid and the alkali/base to form a colourless solution. i.e.
(i) when reacting with nitric(V)acid, the hydroxide shows basic properties. It reacts with the acid to form a simple salt and water only.
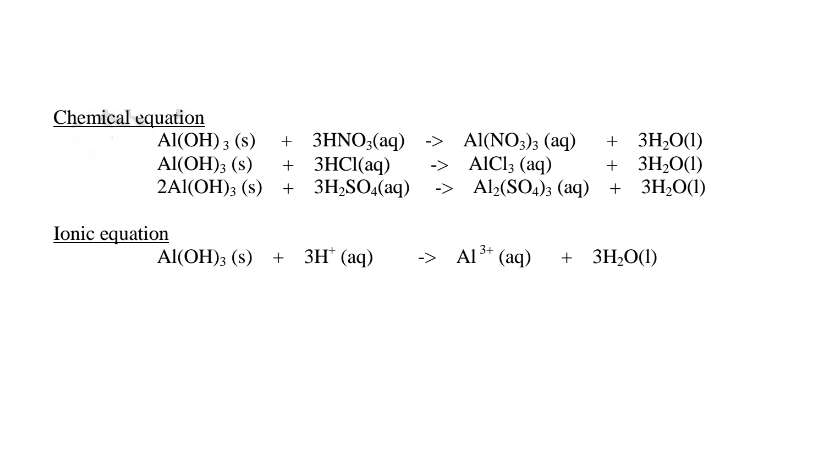
(ii) when reacting with sodium hydroxide, the hydroxide shows acidic properties.
It reacts with a base to form a complex salt.
Chemical equation 1.When aluminium(III) hydroxide is reacted with sodium hydroxide the complex salt is sodium tetrahydroxoaluminate(III) complex salt.
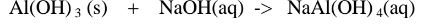
2.When aluminium(Ill) hydroxide is reacted with potassium hydroxide the complex salt is potassium tetrahydroxoaluminate(III) complex salt.
Summary of amphotellic oxides/hydroxides
12.(a) A salt is an ionic compound formed when the cation from a base combine with the anion derived from an acid.
A salt is therefore formed when the hydrogen ions in an acid are replaced wholly/ fully or partially/partly ,directly or indirectly by a metal or armnonium radical.
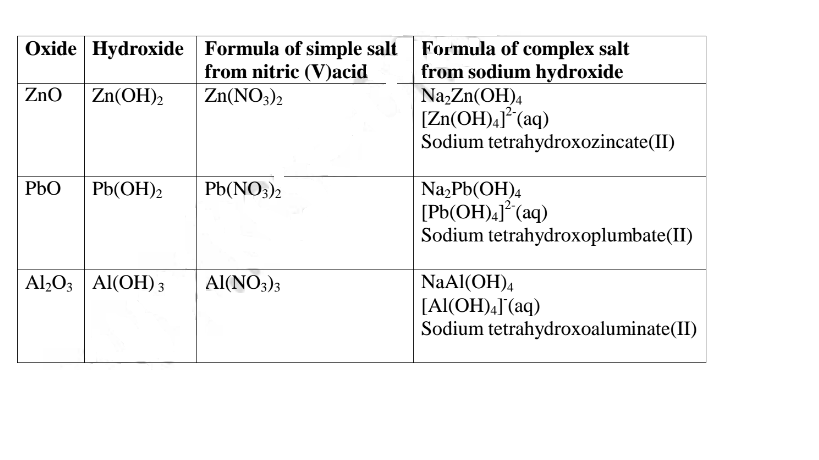
(b) The number of ionizable/replaceable hydrogen in an acid is called basicity of an acid.
Some acids are therefore:
(c) Some salts are normal salts while other are acid salts.
(i)A normal salt is formed when all the ionizable /replaceable hydrogen in an acid is replaced by a metal or metallic /ammonium radical
(ii)An acid salt is fonned when part/portion the ionizable /replaceable hydrogen in an acid is replaced by a metal or metallic /ammonium radical.
Table showing normal and acid salts derived from common acids
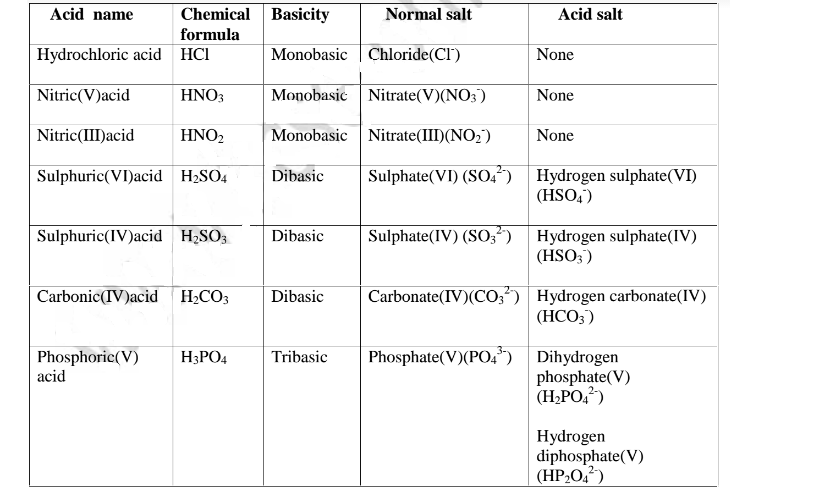
The table below show shows some examples of salts.
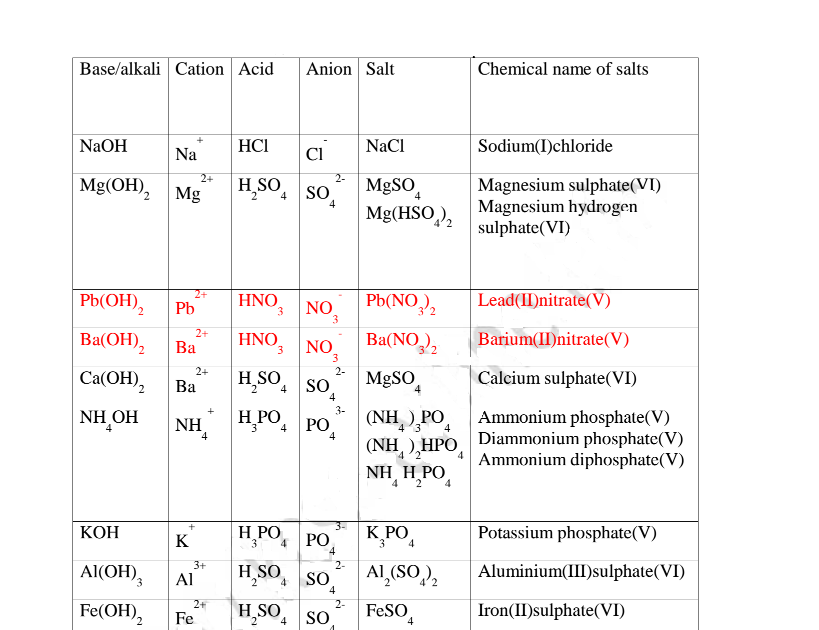
(d) Some salts undergo hygroscopy, deliquescence and efflorescence.
(i) Hygroscopic salts /compounds are those that absorb water from the atmosphere but do not form a solution.
Some salts which are hygroscopic include anhydrous copper(lI)sulphate(VI), anhydrous cobalt(Il)chloride, potassium nitrate(V) common table salt.
(ii)Deliquescent salts /compounds are those that absorb water from the atmosphere and form a solution.
Some salts which are deliquescent include: Sodium nitrate(V),Calcium chloride, Sodium hydroxide, Iron(II)chloride, Magnesium chloride.
(iii)Efflorescent salts/compounds are those that lose their water of crystallization to the atmosphere.
Some salts which efflorescence include: sodium carbonate decahydrate, Iron(II)sulphate(VI)heptahydrate, sodium sulphate (VI)decahydrate.
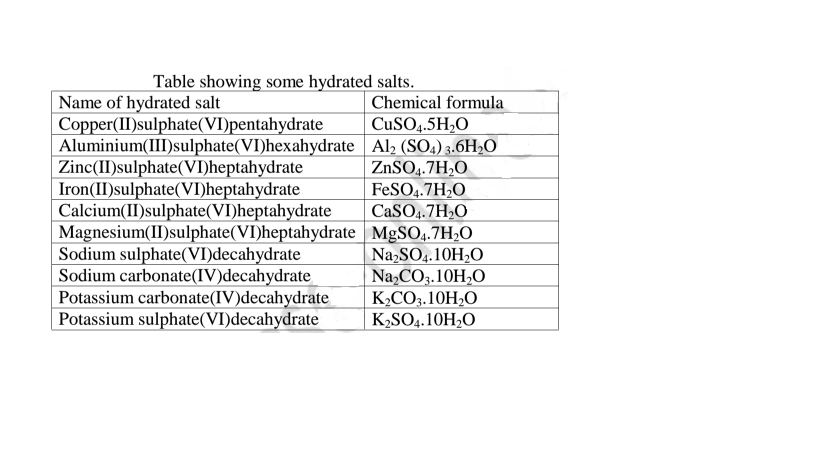
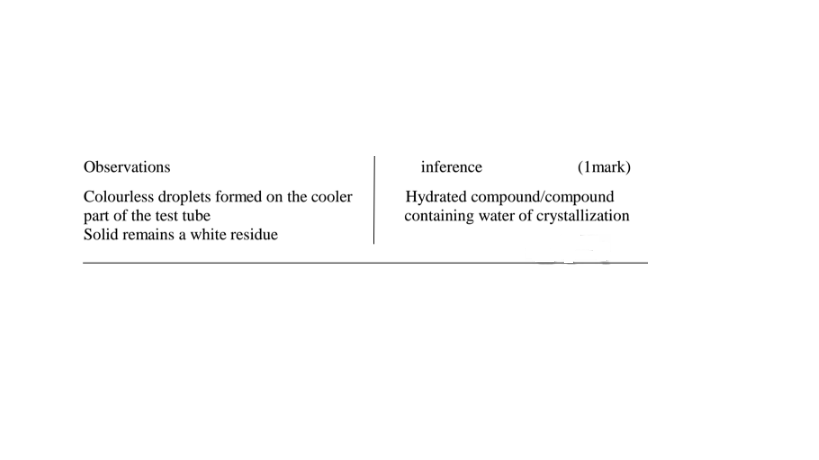
(e)Some salts contain water of crystallization.They are hydrated.Others do not contain water of crystallization. They are anhydrous.
Table showing some hydrated salts.
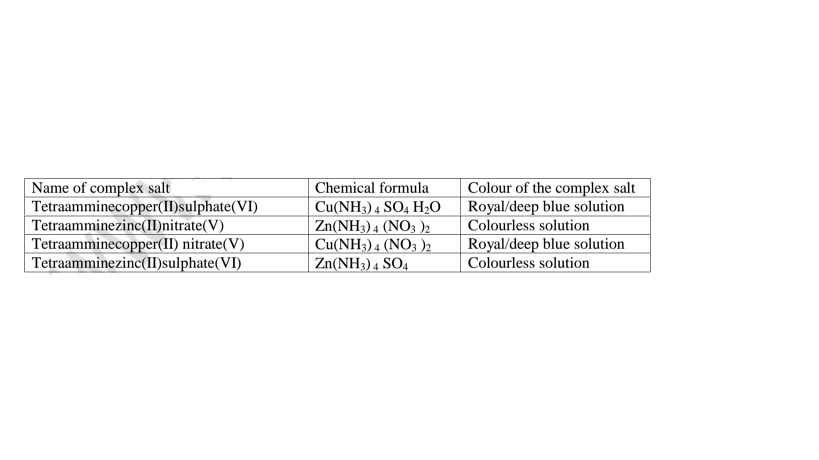
(i)Some salts exist as a simple salt while some as complex salts. Below are some complex salts.
Table of some complex salts
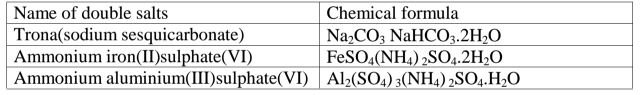
(g)Some salts exist as two salts in one. They are called double salts.
Table of some double salts
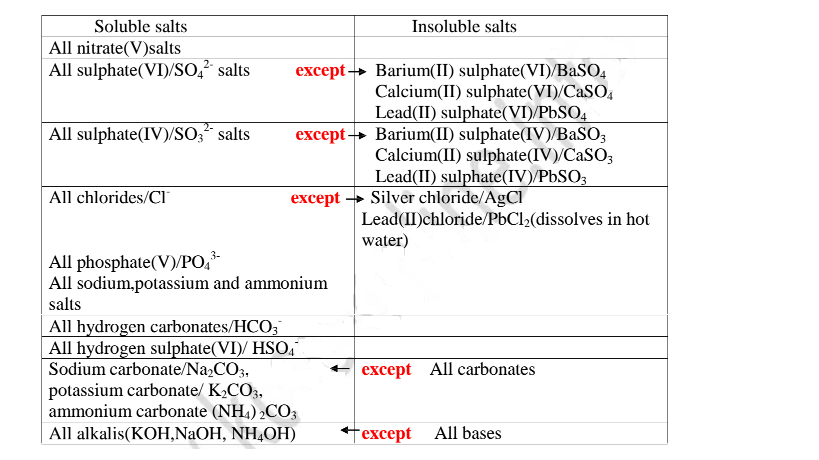
Others do not dissolve in water. They form a suspension/precipitate in water.
Table of solubility of salts
13 Salts can be prepared in a school laboratory by a method that uses its solubility in water.
(a) Soluble salts may be prepared by using any of the following methods:
(i)Direct displacement/reaction of a metal with an acid.
By reacting a metal higher in the reactivity series than hydrogen with a dilute acid,a salt is formed and hydrogen gas is evolved.
Excess of the metal must be used to ensure all the acid has reacted.
When effervescence/bubbling /fizzing has stopped ,excess metal is filtered.
The filtrate is heated to concentrate then allowed to crystallize.
Washing with distilled water then drying between filter papers produces a sample crystal of the salt. i.e.
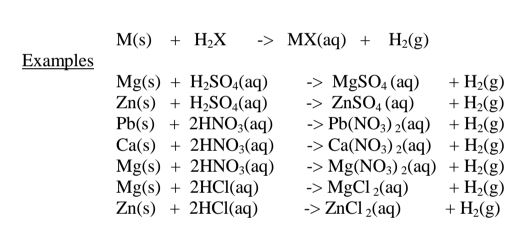
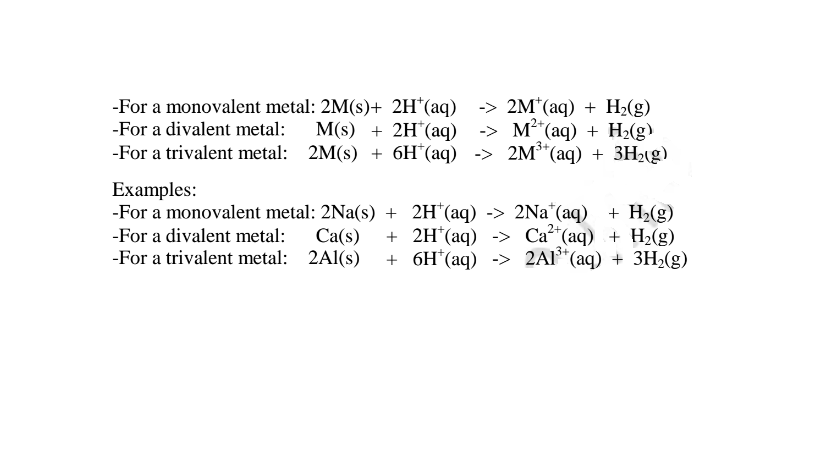
By adding an insoluble base (oxide/hydroxide )to a dilute acid until no more dissolves, in the acid,a salt and water are formed. Excess of the base is filtered off.
The filtrate is heated to concentrate ,allowed to crystallize then washed with distilled water before drying between filter papers e.g.

By adding an excess of a soluble /insoluble carbonate or hydrogen carbonate to a dilute acid, effervescence /fizzing/bubbling out of carbon(lV)oxide gas shows the reaction is taking place.
When effervescence /fizzing/bubbling out of the gas is over, excess of the insoluble carbonate is filtered off. The filtrate is heated to concentrate ,allowed to crystallize then washed with distilled water before drying between filter paper papers e. g.
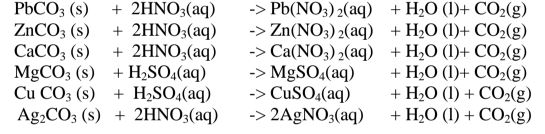
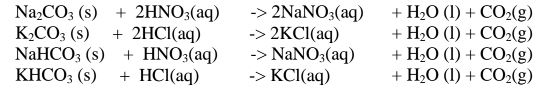
(iv)neutralization/reaction of soluble base/alkali with dilute acid
By adding an acid to a burette into a known volume of an alkali with 2-3 drops of an indicator, the colour of the indicator changes when the acid has completely reacted with an alkali at the end point.
The procedure is then repeated without the indicator .The solution mixture is then heated to concentrate , allowed to crystallize ,washed with distilled water before drying with filter papers. e. g.

(iv)direct synthesis/combination.
When a metal burn in a gas jar containing a non metal , the two directly combine to form a salt. e.g.
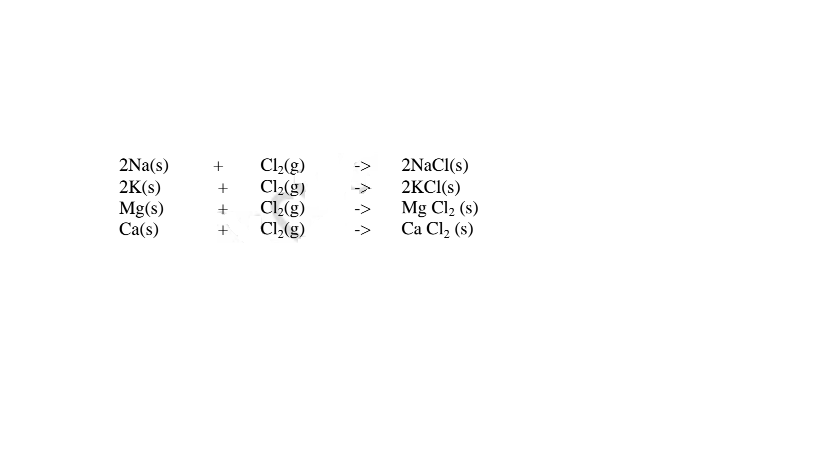
1. Heated aluminium foil reacts with chlorine to form aluminium(lII)chloride that sublimes away from the source of heating then deposited as solid again
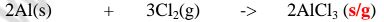
Once formed aluminium(11l)chloride hydrolyses/reacts with water vapour/ moisture present to form aluminium hydroxide solution and highly acidic fumes of hydrogen chloride gas.
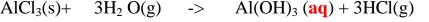
2. Heated iron filings reacts with chlorine to form iron(III)chloride that sublimes away from the source of heating then deposited as solid again
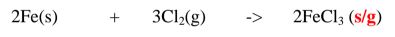
Once formed , aluminium(III)chloride hydrolyses/reacts with Water vapour/ moisture present to form aluminium hydroxide solution and highly acidic fumes of hydrogen chloride gas.
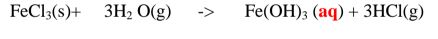
(b)Insoluble salts can be prepared by reacting two suitable soluble salts to form one soluble and one insoluble. This is called double decomposition or precipitation. The mixture is filtered and the residue is washed with distilled Water then dried.

14. Salts may lose their water of crystallization , decompose ,melt or sublime on heating on a Bunsen burner flame.
The following shows the behavior of some salts on heating gently /or strongly in a laboratory school burner:
(a)effect of heat on chlorides
All chlorides have very high melting and boiling points and therefore are not affected by laboratory heating except ammonium chloride. Ammonium chloride sublimes on gentle heating. It dissociate into the constituent ammonia and hydrogen chloride gases on strong heating.
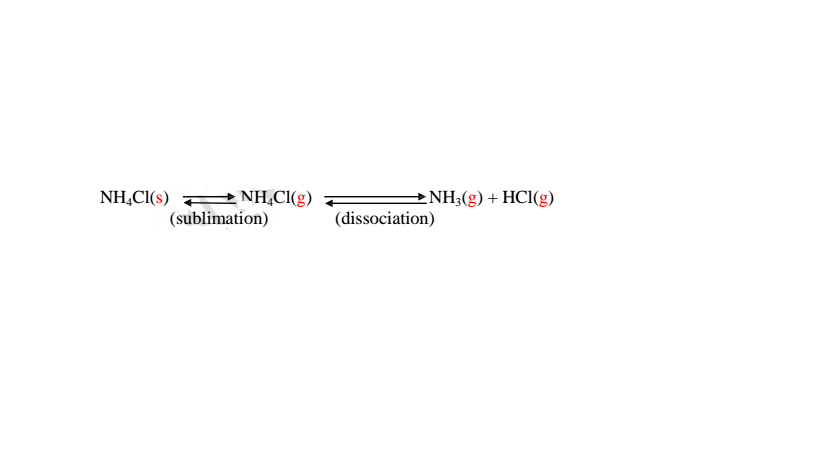
(b)effect of heat on nitrate(V)
(i) Potassium nitrate(V)/KNO3 and sodium nitrate(V)/NaNO3 decompose on heating to form Potassium nitrate(III)/KNO2 and sodium nitrate(III)/NaNO2 and producing Oxygen gas in each case.
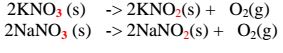
(ii)Heavy metal nitrates(V) salts decompose on heating to form the oxide and a mixture of brown acidic nitrogen(IV)oxide and oxygen gases. e.g.
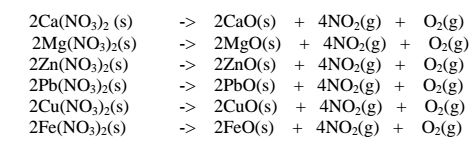
(iii)Silver(I)nitrate(V) and mercury(II) nitrate(V) are lowest in the reactivity series They decompose on heating to form the metal(silver and mercury)and the Nitrogen(IV)oxide and oxygen gas. i.e.
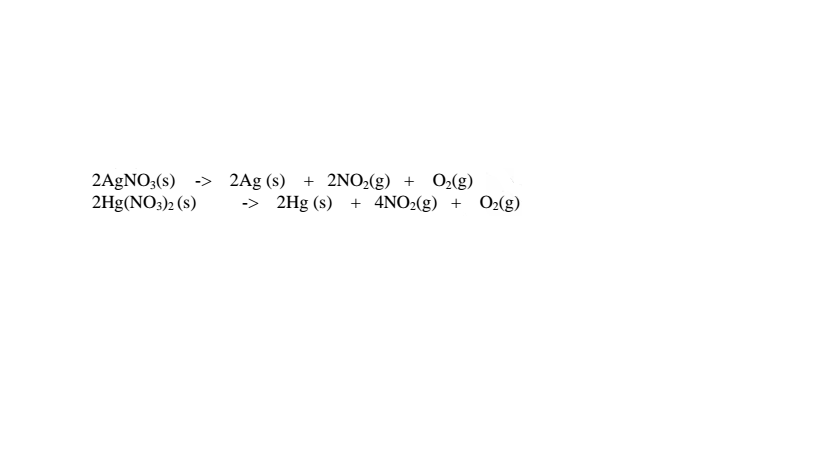
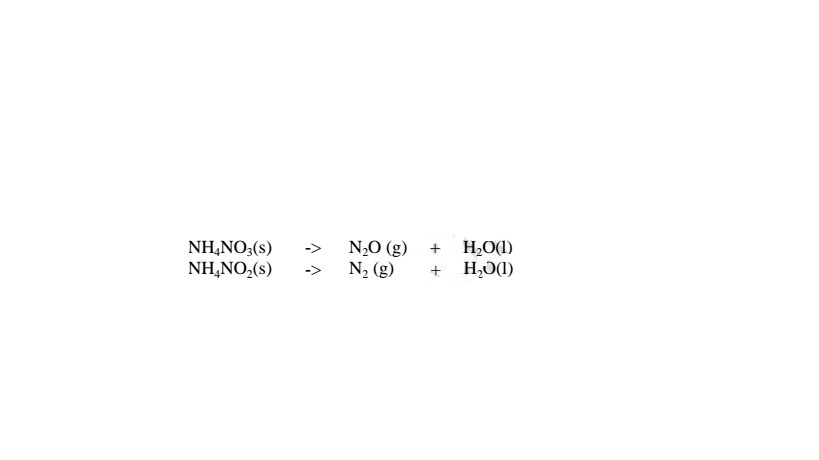
(iv)Ammonium nitrate(V) and Ammonium nitrate(III) decompose on heating to Nitrogen(I)oxide(relights/rekindles glowing splint) and nitrogen gas respectively.Water is also formed.i.e.
(c) effect of heat on nitrate(V)
Only Iron(II)sulphate(VI), Iron(III)sulphate(VI) and copper(II)sulphate(VI) decompose on heating. They form the oxide, and produce highly acidic fumes of acidic sulphur(IV)oxide gas.

(d) effect of heat on carbonates(IV) and hydrogen carbonate(IV).
(i)Sodium carbonate(IV)and potassium carbonate(IV)do not decompose on heating.
(ii)Heavy metal nitrate(IV)salts decompose on heating to form the oxide and produce carbon(IV)oxide gas. Carbon (IV)oxide gas forms a white precipitate when bubbled in lime Water. The White precipitate dissolves if the gas is in excess

(iii)Sodium hydrogen carbonate(IV) and Potassium hydrogen carbonate(IV)decompose on heating to give the corresponding carbonate (IV) and form water and carbon(IV)oxide gas. i.e.
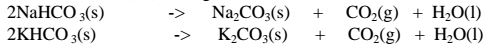
(iii) Calcium hydrogen carbonate (IV) and Magnesium hydrogen carbonate(IV) decompose on heating to give the corresponding carbonate (IV) and form water and carbon(IV)oxide gas. i. e.
15. Salts contain cation(positively charged ion) and anions(negatively charged ion).When dissolved in polar solvents/water.
The cation and anion in a salt is determined/known usually by precipitation of the salt using a precipitating reagent.
The colour of the precipitate is a basis of qualitative analysis of a compound.
16.Qualitative analysis is the process of identifying an unknown compound /salt by identifying the unique qualities of the salt/compound.
It involves some of the following processes.
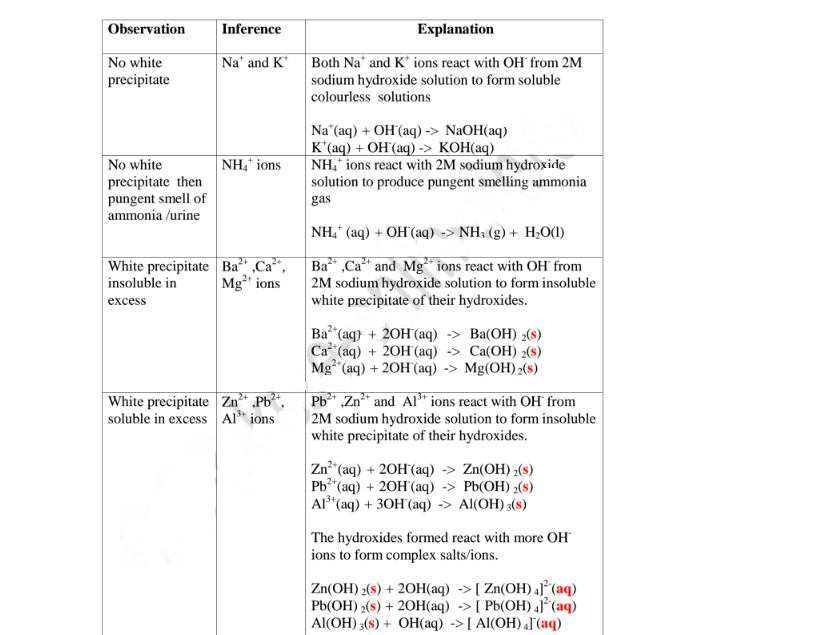
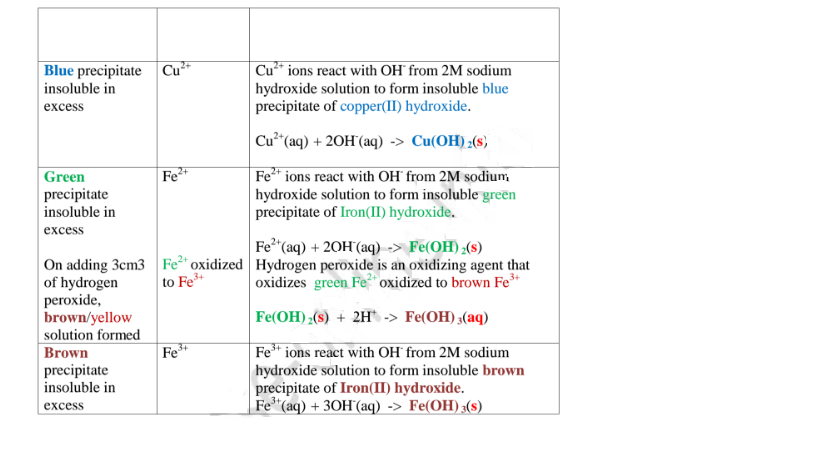
(a)Reaction of cation with sodium/potassium hydroxide solution.
Both sodium/potassium hydroxide solutions are precipitating reagents.
The alkalis produce unique colour of a precipitate/suspension when a few/three drops is added and then excess alkali is added to unknown salt/compound solution.
NB: Potassium hydroxide is not commonly used because it is more expensive than sodium hydroxide.
The table below shows the observations, inferences / deductions and explanations from the following test tube experiments:
Procedure
Put about 2cm3 of MgCl2, CaCl2, AlCl3, NaCl, KCI, FeSO4, Fe2(SO4)3, CuSO4, ZnSO4NH4NO3, Pb(NO3)2, Ba(NO3)2 each into separate test tubes. Add three drops of 2M sodium hydroxide solution then excess (2/3 the length of a standard test tube).
Aqueous ammonia precipitating reagent that can be used to identify the cations present in a salt.
Like NaOH/KOH the OH’ ion in NH4OH react with the cation to form a characteristic hydroxide .
Below are the observations ,inferences and explanations of the reactions of aqueous ammonia with salts from the following test tube reactions.
Procedure
Put about 2cm3 of MgCl2, CaCl2, AlCl3, NaCl, KCI, FeSO4, Fe2(SO4)3, CuSO4, ZnSO4NH4NO3, Pb(NO3)2, Ba(NO3)2 each into separate test tubes.
Add three drops of 2M aqueous ammonia then excess (2/3 the length of a standard test tube).
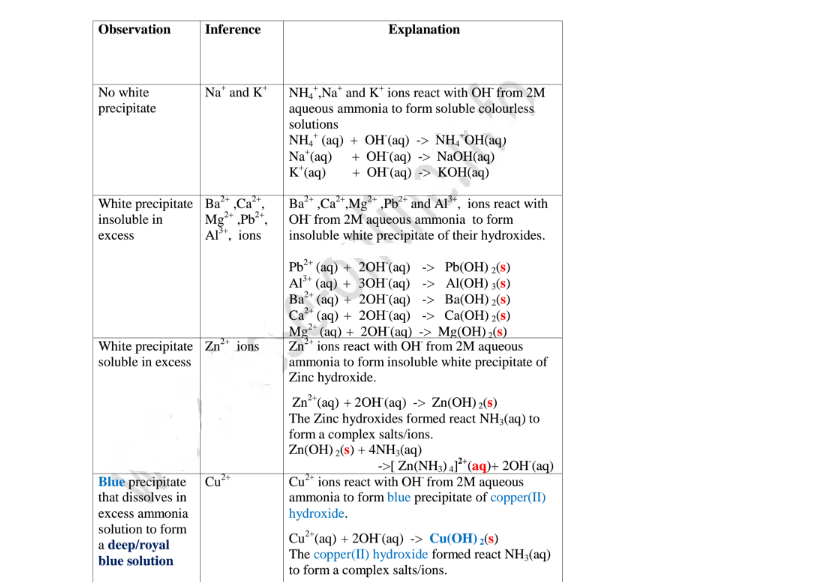
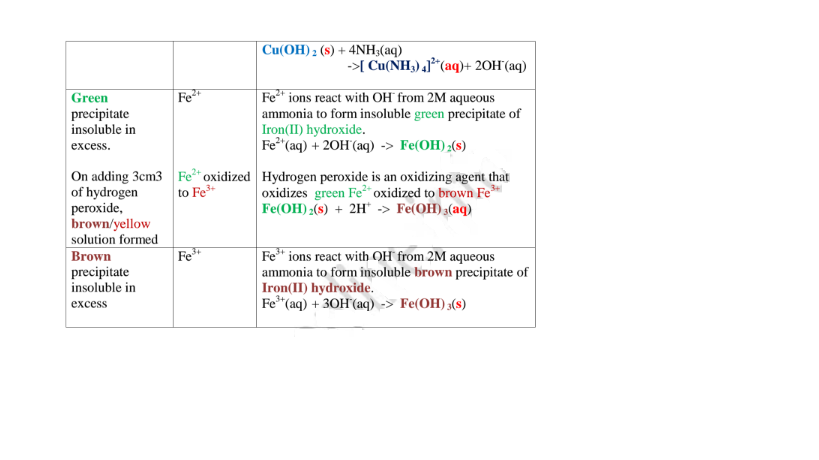
(i) Only Zn2+ ions/salts form a white precipitate that dissolve in excess of both 2M sodium hydroxide and 2M aqueous ammonia.
(ii) Pb2+ and Al3+ ions/salts form a white precipitate that dissolve in excess of 2M sodium hydroxide but not in 2M aqueous ammonia.
(iii) Cu2+ ions/salts form a blue precipitate that dissolve to form a deep/royal blue solution in excess of 2M aqueous ammonia but only blue insoluble precipitate in 2M sodium hydroxide
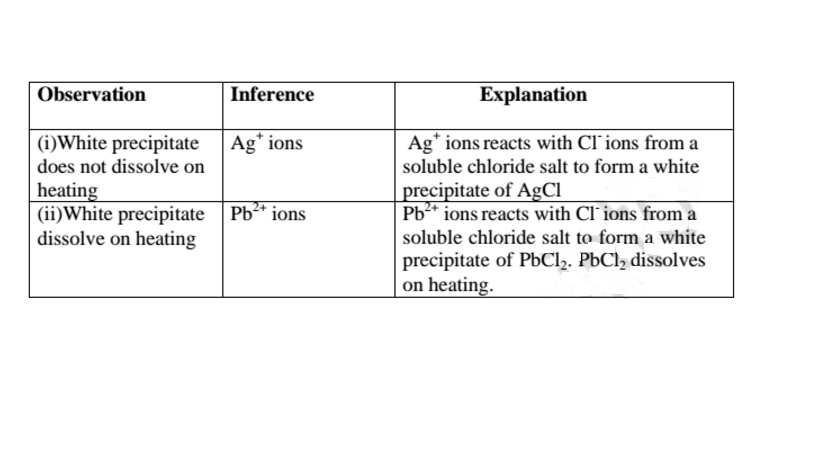
(c)Reaction of cation with Chloride (Cl-)ions
All chlorides are soluble in water except Silver chloride and Lead (II)chloride (That dissolve in hot Water).When a soluble chloride like NaCl, KCI, NH4Cl is added to about 2cm3 of a salt containing Ag+ or Pb2+ ions a White precipitate of AgCl or PbCl2 is formed. The following test tube reactions illustrate the above.
Experiment
Put about 2cm3 of silver nitrate(V) and Lead(II)nitrate(V)solution into separate test tubes. Add five drops of NaCl /KCl / NH4Cl/HCl. Heat to boil.
Note
Both Pb2+ and Al3+ ions forms an insoluble white precipitate in excess aqueous ammonia. A white precipitate on adding Cl' ions/salts shows Pb2+. No white precipitate on adding Cl‘ ions/salts shows Al3+. Adding a chloride/ Cl' ions/salts can thus be used to separate the identity of Al3+ and Pb2+.
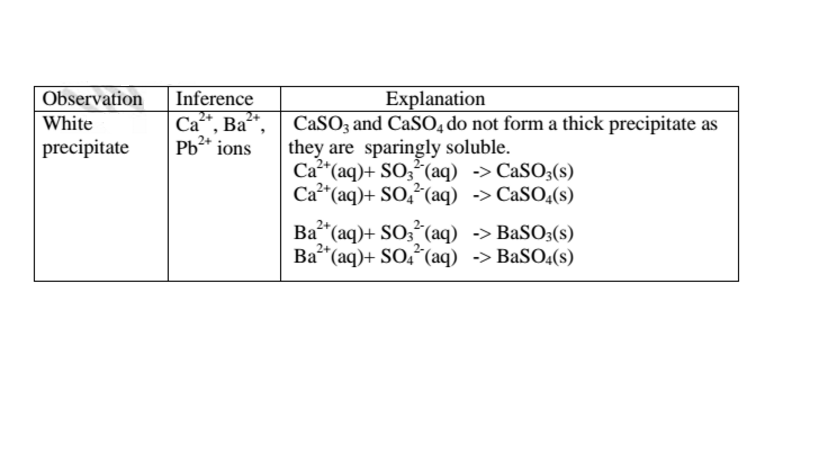
(d)Reaction of cation with sulphate(VI)/SO42‘ and sulphate(IV)/S03} ions
All sulphate(VI) and sulphate(IV)/SO32" ions/salts are soluble/dissolve in water except Calcium sulphate(VI)/CaSO4, Calcium sulphate(IV)/CaSO3, Barium sulphate(VI)/BaSO4, Barium sulphate(IV)/BaSO3, Lead(II) sulphate(VI)/PbSO4 and Lead(II) sulphate(IV)/PbSO3.When a soluble sulphate(VI)/SO42' salt like Na2SO4, HZSO4, (NH4)2SO4 or Na2SO3 is added to a salt containing Ca2+, Pb”, Ba” ions, a white precipitate is formed.
The following test tube experiments illustrate the above.
Procedure
Place about 2cm3 of Ca(NO3)2, Ba(NO3)2, BaCl2 and Pb(NO3)2, in separate boiling tubes. Add six drops of sulphuric(VI)acid /sodium sulphate(VI)/ammonium sulphate(VI)solution. Repeat with six drops of sodium sulphate(IV).
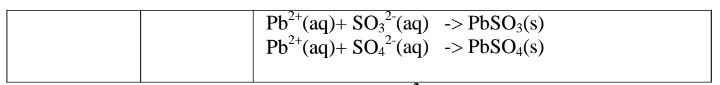
All carbonate salts are insoluble except sodium/potassium carbonate(IV) and ammonium carbonate(IV).
They dissociate /ionize to release CO3 2- ions. CO3 2- ions produce a white precipitate when the soluble carbonate salts is added to any metallic cation.
Procedure
Place about 2cm3 of Ca(NO3)2, Ba(NO3)2, MgCl2 ,Pb(NO3)2 and ZnSO4 in separate boiling tubes.
Add six drops of Potassium /sodium carbonate(IV)/ ammonium carbonate (IV)solution.
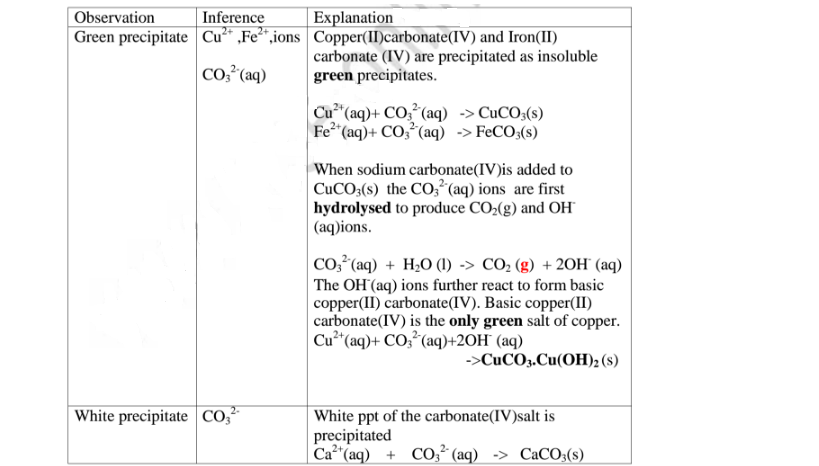
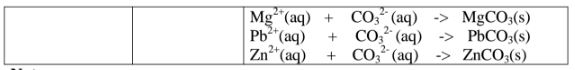
(i)Iron(III)carbonate(IV) does not exist.
(ii)Copper(ll)Carbonate(IV) exist only as the basic CuCO3.Cu(OH) 2
(iii)Both BaCO3 and BaSO; are insoluble White precipitate. If hydrochloric acid is added to the white precipitate;
I. BaCO3 produces CO2 gas. When bubbled/directed into lime water solution,a white precipitate is formed.
II. I. BaSO3 produces SO; gas. When bubbled/directed into orange acidified potassium dichromate(VI) solution, it turns to green/decolorizes acidified potassium manganate(VII).
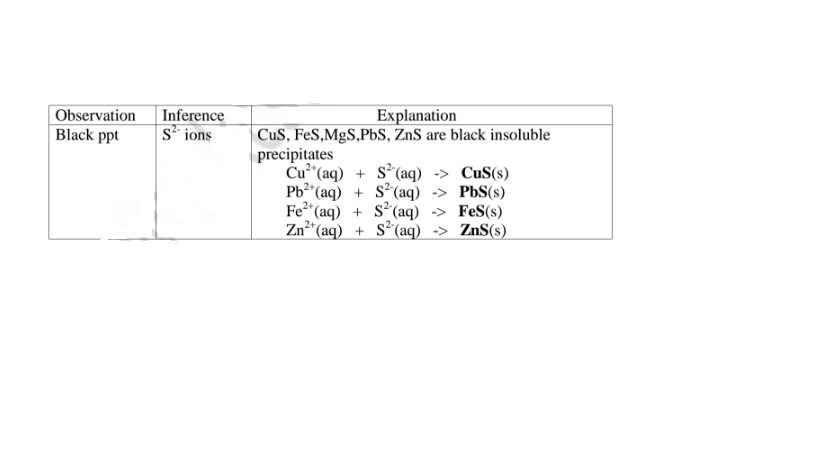
(f) Reaction of cation with sulphide / S2- ions
All sulphides are insoluble black solids/precipitates except sodium sulphide/ Na2S/ potassium sulphide/K2S. When a few/3 drops of the soluble sulphide is added to a metal cation/salt, a black precipitate is formed.
Procedure
Place about 2cm3 of Cu(NO3)2, F eSO4, MgCl2,Pb(NO3)2 and ZnSO4 in separate boiling tubes.
Add six drops of Potassium /sodium sulphide solution.
You are provided with solid Y(aluminium (III)sulphate(VI)hexahydrate).Carry out the following tests and record your observations and inferences in the space provided.

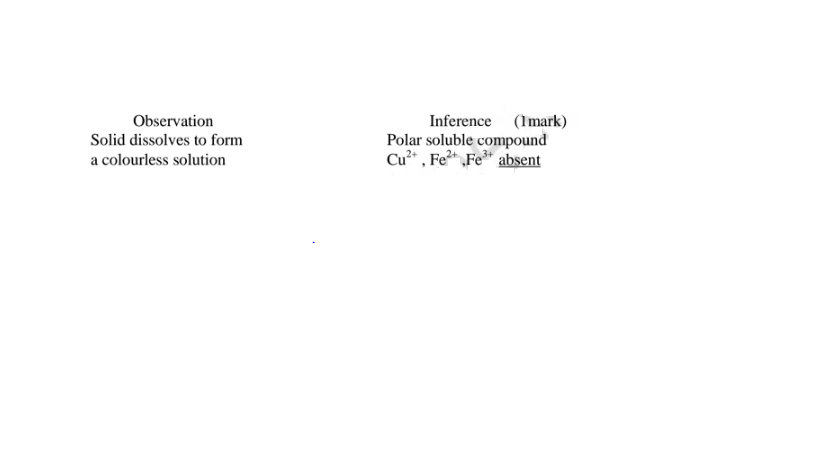
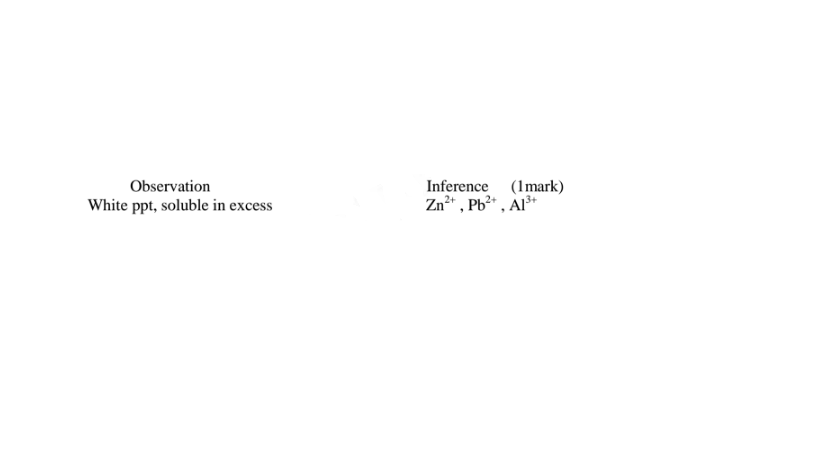

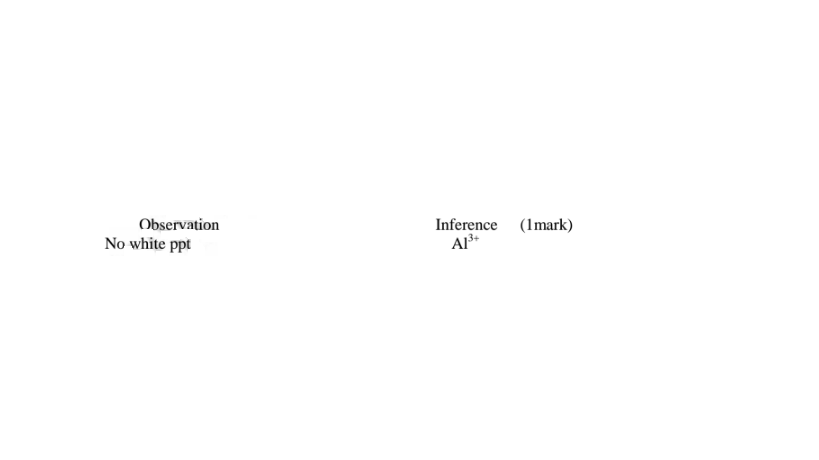
(iv)I.To the fourth portion, add three drops of Lead(II)nitrate(IV)solution. Preserve



(i)From test above, it can be deduced that solid Y is hydrated aluminium(lII)sulphate(VI) solid
(ii)Any ion inferred from an observation below must be derived from previous correct observation and inferences above. e.g.
Al“ in c(m) must be correctly inferred in either/or in c(ii) or c(i)above SO42' in c(iv)III must be correctly inferred in either/or in c(iv)lI or c(iv) above
(iii)Contradiction in observations and inferences should be avoided eg.
“White ppt soluble in excess” to infer presence of Al“ ,Q“ ,Pb3+
(iv)Symbols of elements/ions should be correctly capitalized. e.g.
“SO4'2” is Wrong, “sO42"’ is Wrong, “cuzl” is Wrong.
Sample solutions of salt were labeled as I,II, III and IV. The actual solutions, not in that order are lead nitrate, zinc sulphate potassium chloride and calcium chloride.
a)When aqueous sodium carbonate was added to each sample separately, a white precipitate was formed in I, III and IV only. Identify solution II.
b)When excess sodium hydroxide was added to each sample separately, a white precipitate was formed in solutions III and I only.
Identify solution I
17.When solids/salts /solutes are added to a solvent ,some dissolve to form a solution.
Solute + Solvent -> Solvent
If a solution has a lot of solute dissolved in a solvent ,it is said to be concentrated.
If a solution has little solute dissolved in a solvent ,it is said to be dilute.
There is a limit to how much solute can dissolve in a given /specified amount of solvent/water at a given /specified temperature.
The maximum mass of salt/solid/solute that dissolve in 100g of solvent/water at a specified temperature is called solubility of a salt.
When no more solute can dissolve in a given amount of solvent at a specified temperature, a saturated solution is formed.
For some salts, on heating, more of the salt/solid/solute dissolve in the saturated solution to form a super saturated solution.
The solubility of a salt is thus calculated from the formula
Solubility = Mass of solute/salt/solid x 100 divided by Mass/volume of water/solvent
Practice examples
(a)Calculate the solubility of potassium nitrate(V) if 5.0 g of the salt is dissolved in 50.0cm3 of water.
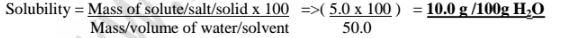
(b)Calculate the solubility of potassium chlorate(V) if 50.0 g of the salt is dissolved in 250.0cm3 of water.
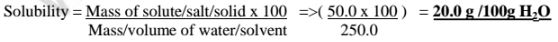
(c)If the solubility of potassium chlorate(V) is 5g/100g H10 at 80°C,how much can dissolve in 5cm3 of Water at 80°C .
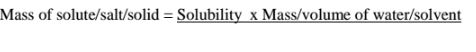
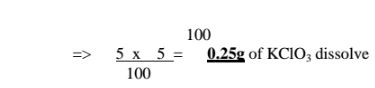
(d)If the solubility of potassium chlorate(V) is 72g/100g H20 at 20°C,how much can saturate 25g of Water at 20°C .
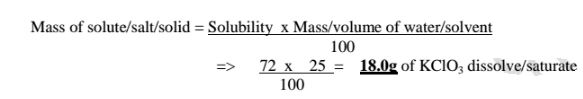
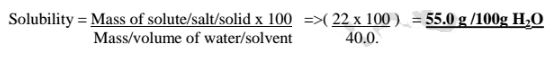
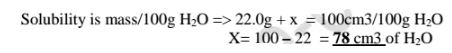
It shows the influence of temperature on solubility of different substances/solids/salts.
Some substances dissolve more with increase in temperature while for others dissolve less with increase in temperature
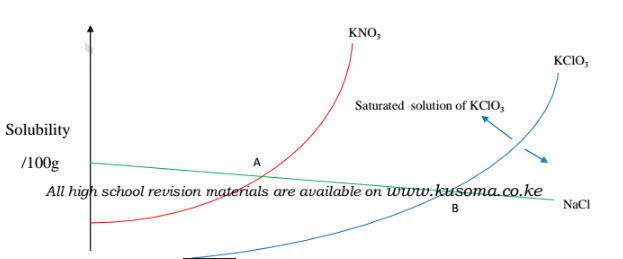
(i)solubility of KNO3 and KClO3 increase with increase in temperature.
(ii)solubility of KNO3 is always higher than that of KCIO3 at any specified temperature.
(iii)solubility of NaCl decrease with increase in temperature.
(iv)NaCl has the highest solubility at low temperature while KCIO3 has the lowest solubility at low temperature.
(v)At point A both NaCl and KNO3 are equally soluble.
(vi)At point B both NaCl and KCIO3 are equally soluble.
(vii) An area above the solubility curve of the salt shows a saturated /supersaturated solution.
(viii) An area below the solubility curve of the salt shows an unsaturated solution.
19.(a) For salts whose solubility increases with increase in temperature, crystals form when the salt solution at higher temperatures is cooled to a lower temperature.
(b) For salts whose solubility decreases with increase in temperature, crystals form when the salt solution at lower temperatures is heated to a higher temperature.
The examples below shows determination of the mass of crystals deposited with changes in temperature.
1.The solubility of KCIO3 at 100°C is 60g/100g water .What mass of KCIO3 will be deposited at:
(i)75 “C if the solubility is now 39g/100g water.
At 100°C = 60.0g
Less at 75°C = - 39.0g
Mass of crystallized out 21.0 g
(i)35 "C if the solubility is now 28 g/ 100g water.
At 100°C = 60.0g
Less at 35°C = - 28.0.0g
Mass of crystallized out = 32.0g 2. KNO3 has a solubility of 42 g/100g water at 20°C.The salt was heated and added 38g more of the solute which dissolved at 100°C. Calculate the solubility of KNO3 at 100°C.
Solubility of KNO3 at 100°C = solubility at 20°C + mass of KNO3 added => 42g + 38g = so KNO3/100 HZQ
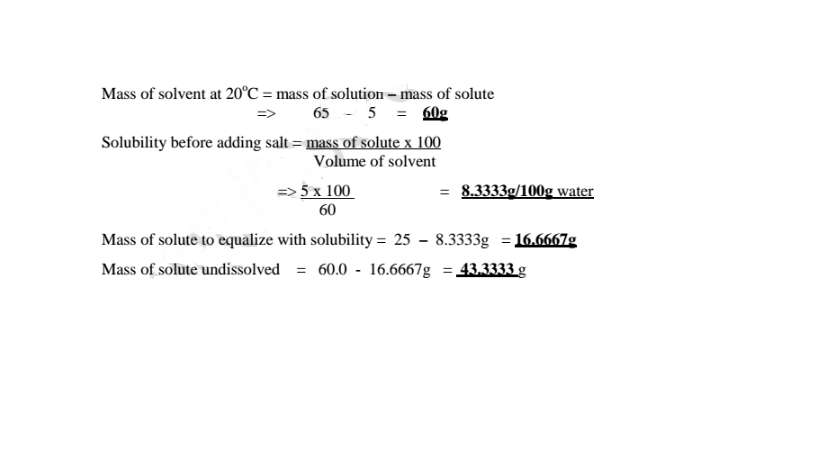
3. A salt solution has a mass of 65g containing 5g of solute. The solubility of this salt is 25g per 100g water at 20°C. 60g of the salt are added to the solution at 20°C.Calculate the mass of the solute that remain undissolved.
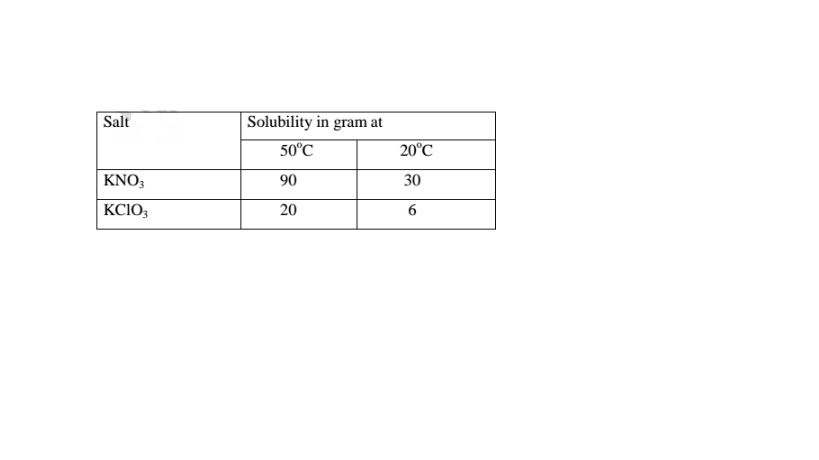
(90 — 30) = 60.0 g of KN03 crystals precipitate
(20 — 6) = 14.0 g of KC103 crystals precipitate
(ii)State the assumption made in (i) above.
Solubility of one salt has no effect on the solubility of the other.
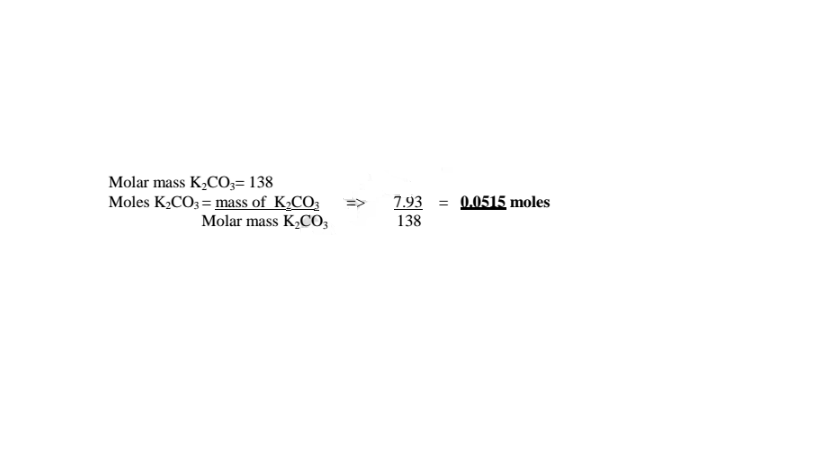
5. 10.0 g of hydrated potassium carbonate (IV) K2CO3.XH20 on heating leave 7.93 of the hydrate.
(a)Calculate the mass of anhydrous salt obtained.
Hydrated on heating leave anhydrous = 7.93 g
(b)Calculate the mass of water of crystallization in the hydrated salt
Mass of Water of crystallization = hydrated - anhydrous -> 10.0 - 7.93 = 2.07 g
(c)How many moles of anhydrous salt are there in 10 of hydrate? (K= 39.0,C=12.0.0= 16.0)
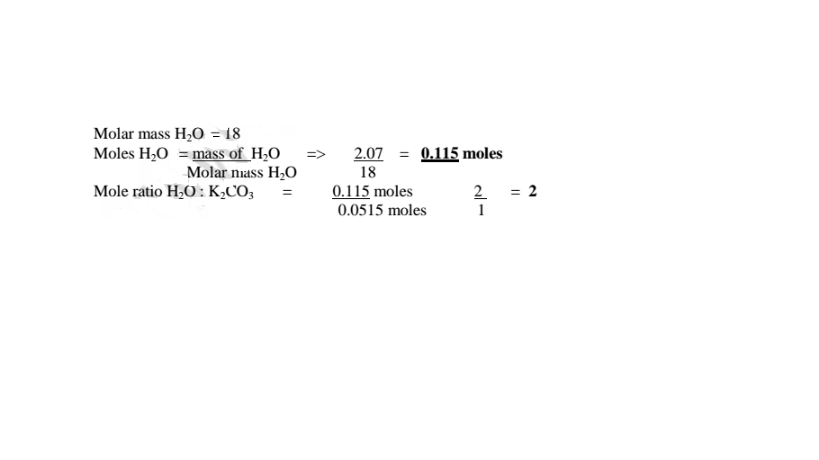
K2C03 .2H20
6. The table below shows the solubility of Potassium nitrate(V) at different temperatures.


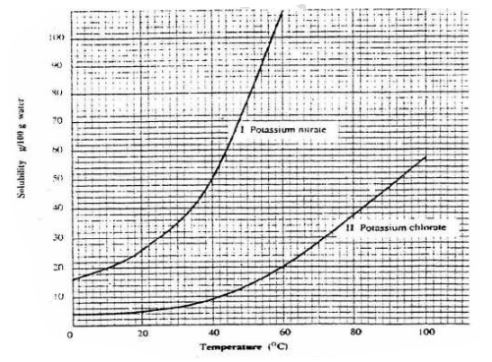
(i)the mass of KNO3 dissolved at:
l.1. 20°C
From a correctly plotted graph = 32g
II. 35°C
From a correctly plotted graph = 57g
III. 55°C
From a correctly plotted graph = 104g
(ii)the temperature at which the following mass of KNO3 dissolved:
I. 22g
From a correctly plotted graph Il3.0°C
II. 30g
From a correctly plotted graph Il7.5°C
III.100g
From a correctly plotted graph I54.5°C
(c)Explain the shape of your graph.
Solubility of KNO3 increase with increase in temperature/More KNO3 dissolve as temperature rises.
(d)Show on the graph the supersaturated and unsaturated solutions.
Above the solubility curve Write; “supersaturated”
Below the solubility curve write; “unsaturated”
(e)From your graph, calculate the amount of crystals obtained when a saturated solution of KNO3 containing 180 g of the salt is cooled from 80°C to
I. 20°C
Solubility before heating = 180 g
Less Solubility after heating(from the graph) = 32 g
Mass of KNO3 crystals = 148 g
II. 35°C
Solubility before heating = 180 g
Less Solubility after heating(from the graph) = 58 g
Mass of KNO3 crystals = 122 g
III. 55°C
Solubility before heating = 180 g
Less Solubility after heating(from the graph) 102 g
Mass of KNO3 crystals = 78 g
7. The table below shows the solubility of salts A and B at various temperatures.
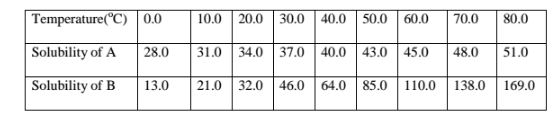
(b)At what temperature are the two salts equally soluble.
The point of intersection of the two curves = 24°C
(c)What happens when a mixture of 100 g of salt B with 100 g if water is heated to 80°C
From the graph, the solubility of B at 80°C is 169 g /100 g water. All the 100 g crystals of B dissolve.
(d)What happens when the mixture in (c) above is then cooled from 50°C to 20°C.
Method I.
Total mass before cooling at 50°C = 100.0 g
(From graph) Solubility/mass after cooling at 20°C = 32.0 g
Mass of crystals deposited = 68.0 g
Method II.
Mass of soluble salt crystals at 50°C added = 100 g
(From graph)Solubility/mass before cooling at 50°C = 85.0 g
Mass of crystals that cannot dissolve at 50°C = 15.0 g
(From graph) Solubility/mass before cooling at 50°C = 85.0 g
(From graph) Solubility/mass after cooling at 20°C = 32.0 g
Mass of crystals deposited after cooling = 53.0 g
Total mass of crystals deposited = 15.0 + 53.0 = 68.0 g
(e)A mixture of 40 g of A and 60 g of B is added to 10 g of water and heated to 70°C.The solution is then allowed to cool to 10°C.Describe clearly what happens.
I.For salt A
Solubility of A before heating = mass of A x 100 divide by Volume of water added => 40 x 100 divide by 10
=400g/100g Water
(Theoretical)Solubility of A before heating = 400 g
Less (From graph ) Solubility of A after heating at 70°C = 48 g
Mass of crystals that can not dissolve at 70°C = 352 g
(From graph ) Solubility of A after heating at 70°C = 48 g
Less (From graph) Solubility of A after cooling to 10°C = 31 g
Mass of crystals that crystallize out on cooling to 10°C = 17 g
Mass of crystals that can not dissolve at 70°C = 352 g
Add Mass of crystals that crystallize out on cooling to 10°C = 17 g
Total mass of A that does not dissolve/crystallize/precipitate = 369 g
I.For salt B
Solubility of B before heating = mass of B X 100 divide by Volume of water added
=> 60 x 100 divide by 10
= 600g/100g Water
(Theoretical)Solubility of B before heating = 600 g
Less (From graph ) Solubility of B after heating at 70°C = 138 g
Mass of crystals that cannot dissolve at 70°C = 462 g
(From graph ) Solubility of B after heating at 70°C = 138 g
Less (From graph) Solubility of B after cooling to 10°C = 21 g
Mass of crystals that crystallize out on cooling to 10°C = 117 g
Mass of crystals that cannot dissolve at 70°C = 462 g
Add Mass of crystals that crystallize out on cooling to 10°C = 117 g
Total mass of A that does not dissolve/crystallize/precipitate = 579 g
(f)State the assumption made in (e)above
Solubility of one salt has no effect on the solubility of the other
8. When 5.0 g of potassium chlorate (V) was put in 10cm3 of water and heated, the solid dissolves. When the solution was cooled , the temperature at which crystals reappear was noted. Another 10cm3 of Water was added and the mixture heated to dissolve then cooled for the crystals to reappear .The table below shows the the results obtained
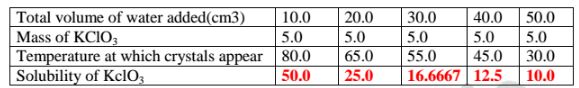
(b)Plot a graph of mass of KCIO3 per 100g water against temperature at which crystals form.
(c)From the graph, show and determine ;
(i)the solubility of KCIO3 at
I. 50°C
From a well plotted graph = 14.5 g KCIO3/100g Water
II. 35°C
From a well plotted graph = 9.0 g K0103/100g water
(ii)the temperature at which the solubility is:
I. 10g/100g water
From a Well plotted graph = 38.0 °C
lI. 45g/100g water
From a well plotted graph = 77.5 °C
(d)Explain the shape of the graph.
Solubility of KCIO3 increase with increase in temperature/more KclO;dissolve as temperature rises.
(e)What happens when 100g per 100g water is cooled to 35.0 °C
Solubility before heating = 100.0 (From the graph) Solubility after cooling = 9.0 Mass of salt precipitated/crystallization = 91.0 g
9. 25.0cm3 of water dissolved various masses of ammonium chloride crystals at different temperatures as shown in the table below.
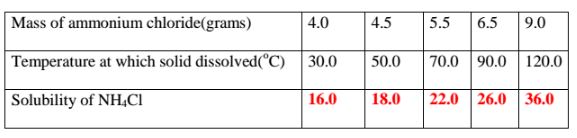
(b)Plot a solubility curve
(c)What happens when a saturated solution of ammonium chloride is cooled from 80°C to 40°C.
(From the graph )Solubility at 80°C = 24.0 g
Less (From the graph )Solubility at 40°C = 16.8 g
Mass of crystallized/precipitated = 7.2 g
20. Solubility and solubility curves are therefore Q
(i) to know the effect of temperature on the solubility of a salt
(ii)to fractional crystallize two soluble salts by applying their differences in solubility at different temperatures.
(iii)determine the mass of crystal that is obtained from crystallization.
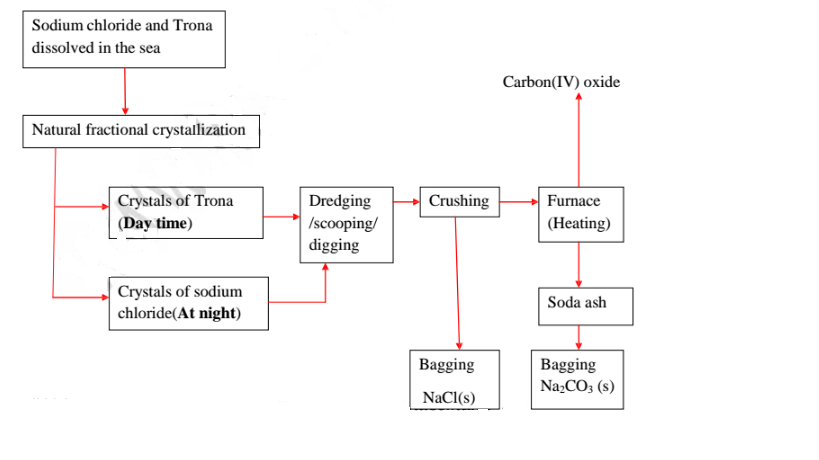
21.Natural fractional crystallization takes place in Kenya/East Africa at:
(i) Lake Magadi during extraction of soda ash(Sodium carbonate) from Trona(sodium sesquicarbonate)
(ii) Ngomeni near Malindi at the Indian Ocean Coastline during the extraction of common salt(sodium chloride).
22.Extraction of soda ash from Lake Magadi in Kenya
Rain water drains underground in the great rift valley and percolate underground where it is heated geothermically.
The hot water dissolves underground soluble sodium compounds and comes out on the surface as alkaline springs which are found around the edges of Lake Magadi in Kenya.
Temperatures around the lake are very high (30-40°C) during the day. The solubility of trona decrease with increase in temperature therefore solid crystals of trona grows on top of the lake (up to or more than 30 metres thick)
A bucket dredger mines the trona which is then crushed ,mixed with lake liquor and pumped to washery plant where it is further refined to a green granular product called CRS.
The CRS is then heated to chemically decompose trona to soda ash(Sodium carbonate)
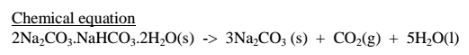
(i) make glass
(ii) for making soapless detergents
(iii) softening hard Water.
(iv) Common salt is collected at night because its solubility decreases with decrease in temperature. It is used as salt lick/feed for animals.
Summary flow diagram showing the extraction of Soda ash from Trona
Oceans are salty.They contain a variety of dissolved salts (about 77% being sodium chloride).
During high tide ,water is collected into shallow pods and allowed to crystallize as evaporation takes place.The pods are constructed in series to increase the rate of evaporation.
At the final pod ,the crystals are scapped together,piled in a heap and washed with brine (concentrated sodium chloride).
It contains MgCl2 and CaCl2 . MgCl2 and CaCl2 are hygroscopic. They absorb water from the atmosphere and form a solution.
This makes table salt damp/wet on exposure to the atmosphere.
24.Some water form lather easily with soap while others do not.
Water which form lather easily with soap is said to be “soft”
Water which do not form lather easily with soap is said to be “hard”
Hardness of water is caused by the presence of Ca2+ and Mg“ ions.
Ca2+ and Mg” ions react with soap to form an insoluble grey /white suspension/precipitate called Scum/ curd. Ca2+ and Mg“ ions in water come from the water sources passing through rocks containing soluble salts of Ca2+ and Mg2+ e. g. Limestone or gypsum
There are two types of water hardness:
(a)temporary hardness of water
(b)permanent hardness of water
(a)temporary hardness of water
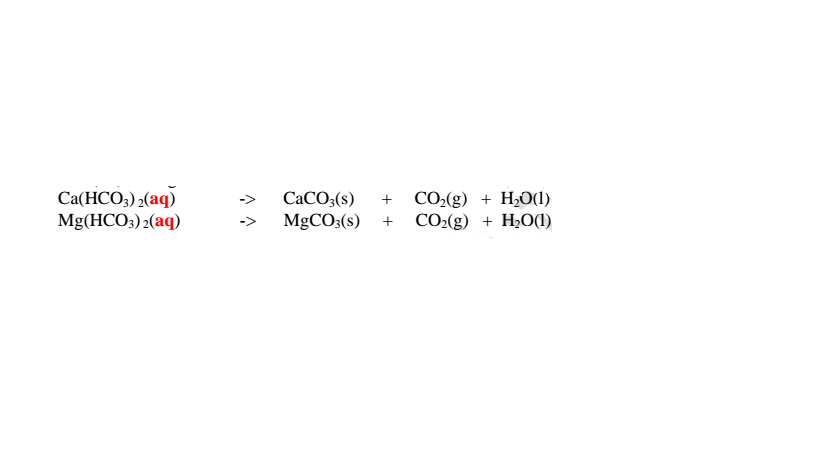
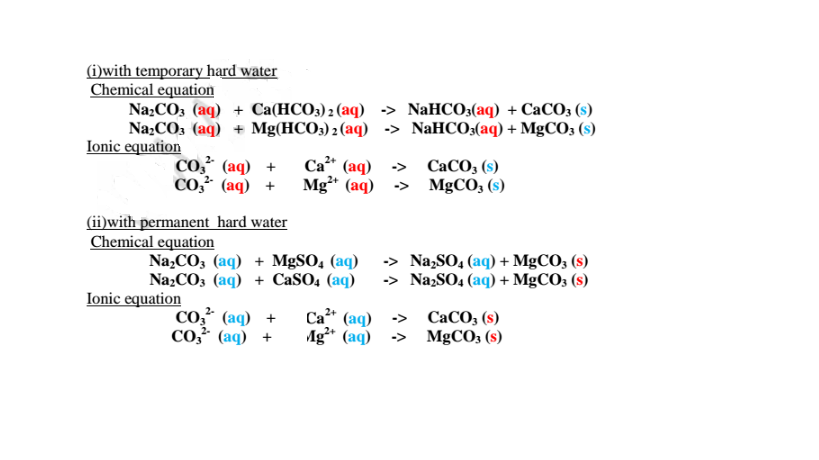
Temporary hardness of water is caused by the presence of dissolved calcium hydrogen carbonate/Ca(HCO3)2 and magnesium hydrogen carbonate/Mg(HCO3)2 When rain water dissolve carbon(IV) oxide from the air it forms waek carbonic(lV) acid i.e.
CO2(g) + H20(l) -> H2CO3(aq)
When carbonic(lV) acid passes through limestone/dolomite rocks it reacts to form soluble salts i.e.
In limestone areas; H2CO3(aq) + CaCO3(s) -> Ca(HCO3)2 (aq)
In dolomite areas; H2CO3(aq) + MgCO3(s) -> Mg(HCO3)2 (aq)
(b)permanent hardness of water
Permanent hardness of water is caused by the presence of dissolved calcium sulphate(VI)/CaSO4 and magnesium sulphate(VI)/Mg S04 Permanent hardness of water is caused by Water dissolving CaSO4 and MgSO4 from ground rocks.
Hardness of water can be removed by the following methods:
(a)Removing temporary hardness of Water
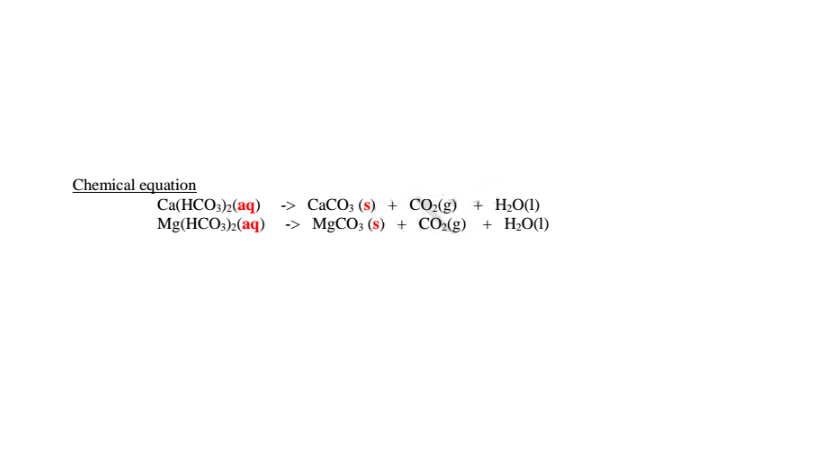
(i)Boiling/heating.
Boiling decomposes insoluble calcium hydrogen carbonate/Ca(HCO3)2 and magnesium hydrogen carbonate/Mg(HCO3)2 to insoluble CaCO3 and MgCO3 that precipitate away. i.e
Since boiling is expensive on a large scale ,a calculated amount of sodium carbonate decahydrate /Na2CO3.10H2O precipitates insoluble Ca2+(aq) and Mg2+(aq) ions as carbonates to remove both temporary and permanent hardness of Water .This a double decomposition reaction where two soluble salts form an insoluble and soluble salt. i.e.
Lime water/calcium hydroxide removes only temporary hardness of water from by precipitating insoluble calcium carbonate(IV).
Chemical equation
Ca(OH)2 (aq) + Ca(HCO3)2(aq) -> 2H20(l) +2CaCO3 (s)
Excess of Lime water/calcium hydroxide should not be used because it dissolves again to form soluble calcium hydrogen carbonate(lV) causing the hardness again.
(iv)Adding aqueous ammonia
Aqueous ammonia removes temporary hardness of water by precipitating insoluble calcium carbonate(IV) and magnesium carbonate(IV)
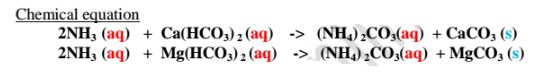
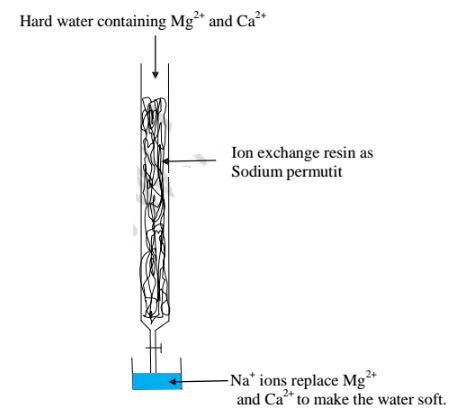
This method involves packing a chamber with a resin made of insoluble complex of sodium salt called sodium permutit.
The sodium pennutit releases sodium ions that are exchanged with Mg2+ and Ca2+ ions in hard Water making the Water to be soft. i.e.
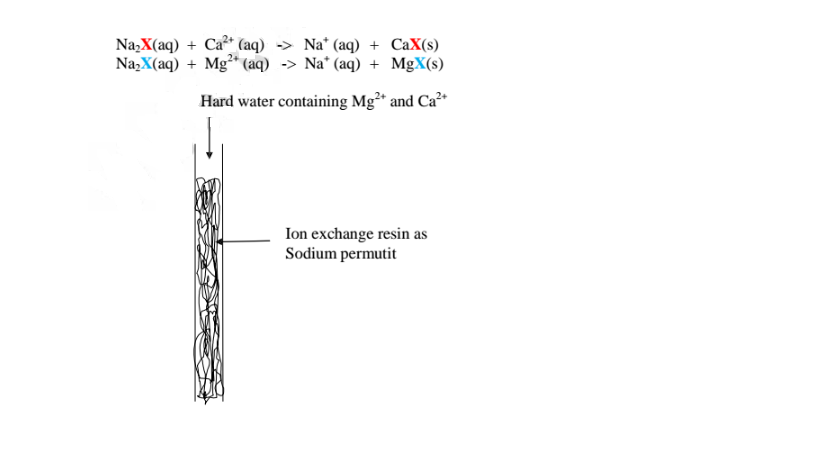
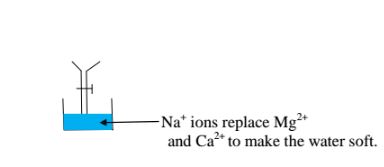
Brine /concentrated sodium chloride solution is passed through the permutit column to regenerated recharge the column again.
This is an advanced ion exchange method of producing deionized water .Deionized water is extremely pure water made only of hydrogen and oxygen only without any dissolved substances.
Deionization involve using the resins that remove all the cations by using:
(i)A cation exchanger which remove /absorb all the cations present in water and leave only H+ ions.
(ii)An anion exchanger which remove /absorb all the anions present in water and leave only OH‘ ions.
The H+(aq) and OH' (aq) neutralize each other to form 1% water.
Chemical equation
H+(aq) + OH- (aq) -> H2O(l)
When exhausted the cation exchanger is regenerated by adding H+(aq) from sulphuric(VI)acid/hydrochloric acid.
When exhausted the anion exchanger is regenerated by adding OH-(aq) from sodium hydroxide.
Advantages of hard water
Hard water has the following advantages:
(i)Ca2+(aq) in hard water are useful in bone and teeth formation
(ii) is good for brewing beer
(iii)contains minerals that cause it to have better /sweet taste
(iv)animals like snails and coral polyps use calcium to make their shells and coral reefs respectively.
(v)processing mineral water
Disadvantages of hard water
Hardness of water:
(i)waste a lot of soap during washing before lather is formed.
(ii)causes stains/blemishes/marks on clothes/garments (iii)causes fur on electric appliances like kettle ,boilers and pipes form decomposition of carbonates on heating .This reduces their efficiency hence more/higher cost of power/electricity.
Sample revision questions
In an experiment, soap solution was added to three separate samples of water. The table below shows the volumes of soap solution required to form lather with 1000cm3 of each sample of water before and after boiling.
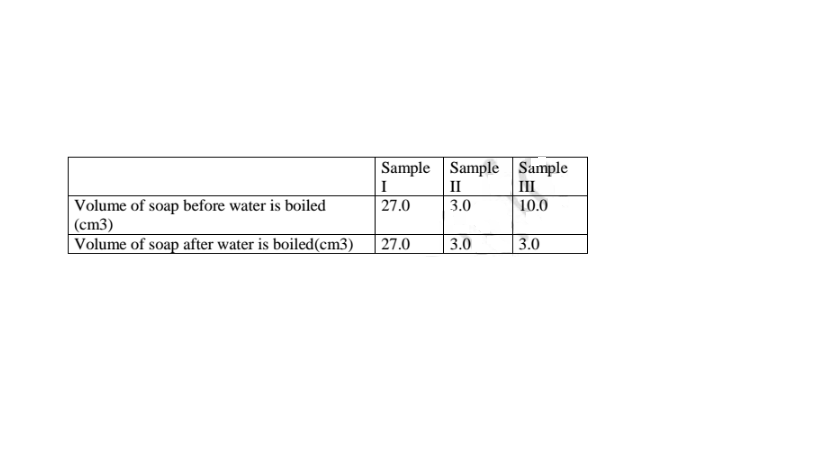
Sample II: Uses little sample of soap .
b) Name the change in the volume of soap solution used in sample III (1 mark)
On heating the sample water become soft because it is temporary hard.
2.Study the scheme below and use it to answer the questions that follow:
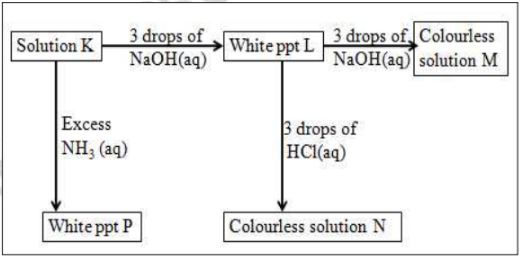
(i)Cation in solution K
A13+
(ii)white ppt L
Al(OH)3
(iii) colourless solution M
[A1(OH)4]-
(iv) colourless solution N
AlCl3
(v)White ppt P
Al(OH)3
(b)Write the ionic equation for the reaction for the formation of:
(i)white ppt L
A13 +(aq) + 3OH- (aq) -> Al(OH)3(s)
(v)White ppt P
Al3+(aq) + 3OH-(aq) -> Al(OH)3(s)
(c)What property is illustrated in the formation of colourless solution M and N Amphotellic
KCSE Revision Notes Form 1 - Form 4 All Subjects
Kenya Scholarships for Undergraduate Students » Kenya Scholarships for Postgraduate Students » Undergraduate Scholarships for Kenyan Students » Kenya Undergraduate Scholarships » Full Undergraduate Scholarships for Kenyans » Kenya Postgraduate Scholarships » Scholarships & Grants » Undergraduate Scholarships » Universities in Kenya » Kenya Universities and Colleges Central Placement Service (KUCCPS) » Colleges in Kenya » KASNEB Registration & Results » Secondary Schools Scholarships in Kenya » Undergraduate & Graduate Scholarships for Kenyans
Scholarships for African Students » Undergraduate Scholarships » African Women Scholarships & Grants » Developing Countries Scholarships » Erasmus Mundus Scholarships for Developing Countries » Fellowship Programs » Funding Grants for NGOs » Government Scholarships » LLM Scholarships » MBA Scholarships » PhD and Masters by Research Scholarships » Public Health Scholarships - MPH Scholarships » Refugees Scholarships » Research Grants » Scholarships and Grants
Scholarships in Australia » Scholarships in Belgium » Scholarships in Canada » Scholarships in Germany » Scholarships in Italy » Scholarships in Japan » Scholarships in Korea » Scholarships in Netherlands » Scholarships in UK » Scholarships in USA
Chemistry kcse questions and answer chemistry paper 3 question and answer kcse chemistry practical kcse chemistry paper 2 2016 kcse chemistry paper 1 2016 chemistry paper 2 questions and answers kcse chemistry notes kcse chemistry paper 2 2014 kcse chemistry paper 1 2013 "Pdf" Revision Questions Chemistry Form 2 "Pdf" Revision Questions Chemistry Form 3 "Pdf" Revision Questions Chemistry Form 4 "Pdf" Revision Questions Chemistry Form Four "Pdf" Revision Questions Chemistry Form One "Pdf" Revision Questions Chemistry Form Three "Pdf" Revision Questions Chemistry Form Two 1 a a KCSE Past Papers 10th Grade Chemistry Questions and Answers 10th Grade Chemistry Test 11th Ncert Chemistry 12th Class Chemistry Book Free Download 2014 KCSE Marking Schemes 2014 Pdf KCSE Past Papers 2015 2015 Chemistry Essay Questions and Answers Form 4 2016 KCSE Papers 2016 KCSE Prediction Questions 2017 Chemistry Hsc Answers 2017 KCSE Prediction Questions 2018 Chemistry KCSE Leakage 2018 Chemistry KCSE Questions 2018 KCSE Busineness Studies 2018 KCSE Exam 2018 KCSE Leakage 2018 KCSE Prediction Questions 2018 KCSE Questions 2019 Chemistry KCSE Leakage 2019 Chemistry KCSE Questions 2019 KCSE Leakage 2019 KCSE Questions 9th Grade Chemistry Study Guide A a a Chemistry Notes a a a Chemistry Notes! a a a ChemistryNotes! A a KCSE Past Papers A Biblical View of Social Justice A Level Chemistry Biological Molecules Questions A Level Chemistry Exam Questions by Topic A Level Chemistry Notes Edexcel A Level Chemistry Notes Xtremepapers A Level Chemistry Past Papers A Level Chemistry Questions and Answers a Level Chemistry Questions and Answers A Level Chemistry Questions and Answers (Pdf) A Level Chemistry Questions and Answers Pdf A Level Chemistry Questions by Topic Kidney Questions With Markschemes A Level Chemistry Revision A Level Chemistry Revision Edexcel A Level Chemistry Revision Guide A Level Chemistry Revision Notes A Level Chemistry Revision Notes Pdf A Level Chemistry Textbook Pdf A Level Chemistry Year 1 / as Aqa Exam Questions by Topic A Level Edexcel Notes a* Chemistry aa Chemistry Form 3 Questions and Answers Advance KCSE Past Papers Advance-africa.com KCSE Rev Quiz Advantages and Disadvantages. All Chemistry Essays All Chemistry Notes for Senior Two All KCSE Past Papers Chemistry With Making Schemes All Marking Schemes Questions and Answers All Past K.c.s.e Questions With Answers Alliance Mocks 2017 Ap Bio Quizzes Ap Chemistry 1 Textbook Pdf Ap Chemistry Essay Questions and Answers Are Sourced From KNEC. As Level Chemistry Notes Atika Chemistry Notes Atika School Chemistry Notes B/s Book 2 Notes Basic Chemistry Books Pdf basic Chemistry Interview Questions and Answers Pdf Basic Chemistry Interview Questions and Answers Pdf Basic Chemistry Pdf Basic Chemistry Questions and Answers Basic Chemistry Questions and Answers Pdf Bbc Bitesize Chemistry Ks3 Bihar Board Chemistry Objective Answer 2017 Bihar Board Chemistry Objective Answer 2018 Bio Answers Bio Quesions Chemistry 0478 Chemistry 101 Chemistry 12th Chemistry 12th Class Notes Pdf Chemistry 2019 Syllabus Chemistry All KCSE Short Notes Chemistry Answers Chemistry Answers Online Free Chemistry Answers Quizlet Chemistry Bk 2 Notes Chemistry Book 1 Chemistry Book 1 Notes Chemistry Book 2 Chemistry Book 2 Notes Chemistry Book 3 Chemistry Book 3 KLB Chemistry Book 3 Notes Chemistry Book 4 Chemistry Book 4 Notes Chemistry Book 4 Pdf Chemistry Book for Class 11 Chemistry Book Four Chemistry Book Four Notes Chemistry Book One Chemistry Book One Notes Chemistry Book Pdf Free Download Chemistry Book Three Chemistry Book Three Notes Chemistry Book Three Pdf Chemistry Book Two Chemistry Book Two Notes Chemistry Books Form Three Chemistry Bowl Chemistry Study Guide Chemistry Bowl Questions Chemistry Chemistry Bowl Questions Earth Chemistry Chemistry Bowl Questions Math Chemistry Bowl Questions Middle School Chemistry Brekthrough Form Two Notes Chemistry Class 12 Ncert Solutions Chemistry Class 12 Pdf Chemistry Communication Syllabus Chemistry Diagram Software Chemistry Diagrams for Class 11 Chemistry Diagrams for Class 12 Chemistry Diagrams for Class 9 Chemistry Diagrams for Class-10 Chemistry Diagrams in Form 1 Chemistry Diagrams in Form 2 Chemistry Diagrams in Form 3 Chemistry Diagrams in Form 4 Chemistry Diagrams Pdf Chemistry Diagrams to Label Chemistry Essay Questions and Answers Chemistry Essay Questions and Answers 2018 Chemistry Essay Questions and Answers Form 1 Chemistry Essay Questions and Answers Form 2 Chemistry Essay Questions and Answers Form 3 Chemistry Essay Questions and Answers Form 4 Chemistry Essay Questions and Answers Form 4 Pdf Chemistry Essay Questions and Answers Pdf Chemistry Essay Revision Q Chemistry Essays and Answers Chemistry Essays Form One to Form Four Chemistry Essays Form One to Form Three Chemistry Essays KCSE Chemistry Essays Pdf Chemistry Exam 1 Multiple Choice Chemistry Exam 2 Advance Chemistry Exam 2 Test Chemistry Exam 2016 Chemistry Exam Form Four Chemistry Exam Form One Chemistry Exam Form Three Chemistry Exam Form Two Chemistry Exam Practice Test Chemistry Exam Questions Chemistry Exam Questions and Answers Chemistry Exam Questions and Answers Pdf Chemistry Exam Study Guide Chemistry Exams Chemistry Excretion Notes Chemistry Exercise Form 4 With Answers Chemistry Final Exam Answer Key Chemistry Final Exam Answer Key 2016 Chemistry Final Exam Answer Key 2017 Chemistry Final Exam Answers 2018 Chemistry Final Exam Answers 2019 Chemistry Final Exam Questions and Answers Chemistry Fom 1 Notes Chemistry Fom 2 Notes Chemistry Fom 3 Notes Chemistry Fom 4 Notes Chemistry Form 1 Chemistry Form 1 & 2 and Answers Chemistry Form 1 and 2 Essays Chemistry Form 1 and 2 Essays Questions and Answers Chemistry Form 1 Chapter 1 Chemistry Form 1 Diagrams Chemistry Form 1 Exams Chemistry Form 1 Mid Year Exam Chemistry Form 1 Notes Chemistry Form 1 Notes and Questions Chemistry Form 1 Notes Download Chemistry Form 1 Notes Free Download Chemistry Form 1 Notes GCSE Chemistry Form 1 Notes KCSE-kcse Chemistry Form 1 Notes Pdf Chemistry Form 1 Notes Pdf Download Chemistry Form 1 Past Papers Chemistry Form 1 Pdf Chemistry Form 1 Pressure Chemistry Form 1 Question Papers Chemistry Form 1 Questions Chemistry Form 1 Questions and Answers Chemistry Form 1 Questions and Answers Pdf Chemistry Form 1 Quiz Chemistry Form 1 Revision Questions Chemistry Form 1 Summary Notes Chemistry Form 1 Syllabus Chemistry Form 1 Work Chemistry Form 1-4 Notes Chemistry Form 2 Chemistry Form 2 Chapter 1 Chemistry Form 2 Chapter 2 Chemistry Form 2 Diagrams Chemistry Form 2 Exam Paper 2014 Chemistry Form 2 Exams Chemistry Form 2 Notes Chemistry Form 2 Notes and Questions Chemistry Form 2 Notes GCSE Chemistry Form 2 Notes KCSE-kcse Chemistry Form 2 Notes Pdf Chemistry Form 2 Notes Pdf Download Chemistry Form 2 Past Papers Chemistry Form 2 Pdf Chemistry Form 2 Question Papers Chemistry Form 2 Questions Chemistry Form 2 Questions and Answers Chemistry Form 2 Questions and Answers Pdf Chemistry Form 2 Quiz Chemistry Form 2 Revision Notes Chemistry Form 2 Salts Chemistry Form 2 Structure and Bonding Chemistry Form 2 Summary Notes Chemistry Form 2 Syllabus Chemistry Form 2 Work Chemistry Form 3 Chemistry Form 3 and 4 Essays Chemistry Form 3 and 4 Essays Questions and Answers Chemistry Form 3 Chapter 3 Chemistry Form 3 Classification Chemistry Form 3 Diagrams Chemistry Form 3 Ecology Chemistry Form 3 Exams Chemistry Form 3 Notes Chemistry Form 3 Notes and Questions Chemistry Form 3 Notes GCSE Chemistry Form 3 Notes KCSE-kcse Chemistry Form 3 Notes Pdf Chemistry Form 3 Notes Pdf Download Chemistry Form 3 Notes Topic 1 Chemistry Form 3 Past Papers Chemistry Form 3 Pdf Chemistry Form 3 Question Papers Chemistry Form 3 Questions Chemistry Form 3 Questions and Answers Chemistry Form 3 Questions and Answers Pdf Chemistry Form 3 Questions and Answers Term 3 Chemistry Form 3 Questions and Answers+pdf Chemistry Form 3 Quiz Chemistry Form 3 Revision Notes Chemistry Form 3 Revision Questions Chemistry Form 3 Summary Notes Chemistry Form 3 Syllabus Chemistry Form 3 Syllabus Pdf Chemistry Form 3 Topics Chemistry Form 3 Work Chemistry Form 4 Chemistry Form 4 All Chapter Chemistry Form 4 Chapter 1 Conversion of Units Chemistry Form 4 Chapter 1 Exercise Chemistry Form 4 Chapter 1 Exercise and Answers Chemistry Form 4 Chapter 1 Exercise Pdf Chemistry Form 4 Chapter 1 Mind Map Chemistry Form 4 Chapter 2 Chemistry Form 4 Chapter 2 Exercise and Answers Chemistry Form 4 Chapter 2 Exercise Pdf Chemistry Form 4 Chapter 2 Experiment Chemistry Form 4 Chapter 2 Formula Chemistry Form 4 Chapter 2 Mind Map Chemistry Form 4 Chapter 2 Momentum Chemistry Form 4 Chapter 2 Notes Pdf Chemistry Form 4 Chapter 2 Objective Questions and Answers Chemistry Form 4 Chapter 2 Paper 2 Chemistry Form 4 Chapter 2 Slideshare Chemistry Form 4 Chapter 3 Chemistry Form 4 Chapter 3 Questions and Answers Chemistry Form 4 Chapter 4 Chemistry Form 4 Chapter 4 Notes Pdf Chemistry Form 4 Chapter 5 Light Questions and Answers Chemistry Form 4 Chapter 5 Notes Pdf Chemistry Form 4 Diagrams Chemistry Form 4 Exam Paper 1 Chemistry Form 4 Exams Chemistry Form 4 Exercise Chemistry Form 4 Exercise Pdf Chemistry Form 4 Module With Answer Chemistry Form 4 Note Chemistry Form 4 Notes Chemistry Form 4 Notes (Pdf) Chemistry Form 4 Notes All Chapter Pdf Chemistry Form 4 Notes and Questions Chemistry Form 4 Notes Chapter 1 Chemistry Form 4 Notes Chapter 2 Chemistry Form 4 Notes Chapter 3 Chemistry Form 4 Notes Download Chemistry Form 4 Notes Free Download Chemistry Form 4 Notes GCSE Chemistry Form 4 Notes KCSE-kcse Chemistry Form 4 Notes Pdf Chemistry Form 4 Notes Pdf Download Chemistry Form 4 Paper 2 Questions and Answers Chemistry Form 4 Past Papers Chemistry Form 4 Question Papers Chemistry Form 4 Questions Chemistry Form 4 Questions and Answers Chemistry Form 4 Questions and Answers Pdf Chemistry Form 4 Quiz Chemistry Form 4 Revision Notes Chemistry Form 4 Schemes of Work Chemistry Form 4 Summary Notes Chemistry Form 4 Syllabus Chemistry Form 4 Textbook Pdf Chemistry Form 4 Work Chemistry Form 5 Chapter 1 Exercise and Answers Chemistry Form 5 Chapter 1 Notes Pdf Chemistry Form 5 Chapter 2 Notes Pdf Chemistry Form 5 Chapter 2 Slideshare Chemistry Form 5 Chapter 3 Notes Pdf Chemistry Form 5 Notes Pdf Chemistry Form Four Book Chemistry Form Four Notes Chemistry Form Four Notes and Questions Chemistry Form Four Notes GCSE Chemistry Form Four Notes Pdf Chemistry Form Four Past Papers Chemistry Form Four Questions Chemistry Form Four Questions and Answers Chemistry Form Four Questions and Answers Pdf Chemistry Form Four Quiz Chemistry Form Four Study Notes Chemistry Form Four Syllabus Chemistry Form Four Topic 2 Chemistry Form Four Topic 4 Chemistry Form Four Topics Chemistry Form Four Work Chemistry Form One Chemistry Form One Book Chemistry Form One Book Pdf Chemistry Form One Download Topic 1 Upto 3 Chemistry Form One Exam Chemistry Form One Notes Chemistry Form One Notes and Questions Chemistry Form One Notes GCSE Chemistry Form One Notes Pdf Chemistry Form One Pdf Chemistry Form One Questions Chemistry Form One Questions and Answers Chemistry Form One Questions and Answers Pdf Chemistry Form One Questions and Their Answers Chemistry Form One Quiz Chemistry Form One Revision Question Chemistry Form One Schemes of Work Chemistry Form One Study Notes Chemistry Form One Syllabus Chemistry Form One Term Three Test Chemistry Form One to Three Notes Chemistry Form One Work Chemistry Form Three Chemistry Form Three Book Chemistry Form Three Notes Chemistry Form Three Notes and Questions Chemistry Form Three Notes GCSE Chemistry Form Three Questions and Answers Chemistry Form Three Questions and Answers Pdf Chemistry Form Three Quiz Chemistry Form Three Reproduction Chemistry Form Three Reproduction. Chemistry Form Three Study Notes Chemistry Form Three Work Chemistry Form Three-questions and Answers Chemistry Form Two Chemistry Form Two Book Chemistry Form Two Diagrams Chemistry Form Two Notes Chemistry Form Two Notes and Questions Chemistry Form Two Notes GCSE Chemistry Form Two Notes Pdf Chemistry Form Two Notes-pdf Chemistry Form Two Pdf Chemistry Form Two Questions Chemistry Form Two Questions and Answers Chemistry Form Two Questions and Answers Pdf Chemistry Form Two Quiz Chemistry Form Two Study Notes Chemistry Form Two Topics Chemistry Form Two Work Chemistry Form Two,schemes of Work Chemistry Form2 Chemistry Form2 Textbook Chemistry Game Form Four Question End Answers Chemistry Grade 10 Exam Papers Chemistry Hsc Pdf Chemistry Human Reproduction Video Chemistry IGCSE Past Papers Xtremepapers Chemistry K.c.s.e 2017 Chemistry KCSE Chemistry KCSE 2016 Chemistry KCSE 2017 Chemistry KCSE 2017 Paper 1 Chemistry KCSE Past Papers Chemistry KCSE Questions Chemistry KCSE Questions and Answer Chemistry KCSE Quizzes & Answers Chemistry KCSE Revision Chemistry KCSE Revision Notes Chemistry KCSE Setting Questions Form One and Two Chemistry Ksce 2015 Chemistry Last Year K.c.s.e Questions Chemistry Lesson Plan Form Two Chemistry Made Familiar Chemistry Mcq for Class 11 Chemistry Mcq for Class 12 Chemistry Mcq for Competitive Exams Chemistry Mcq for Competitive Exams Pdf Chemistry Mcq for Neet Pdf Chemistry Mcq for Ssc Chemistry Mcq Questions With Answers Chemistry Mcq With Answers Pdf Chemistry Mcqs for Class 12 Pdf Chemistry Mcqs With Answers Pdf Chemistry Mid Familia Form One Chemistry Mock Papers Chemistry Module Form 5 Chemistry Multiple Choice Questions and Answers Cxc Chemistry Multiple Choice Questions and Answers Pdf Chemistry Multiple Choice Questions With Answers Pdf Chemistry Note Chemistry Note Form Two All Chapters Chemistry Notes Chemistry Notes and Guestion and Answear Chemistry Notes and Syllabus Chemistry Notes Class 10 Chemistry Notes for Class 11 Pdf Chemistry Notes for Class 12 Pdf Chemistry Notes for High School Students Chemistry Notes for IGCSE 2014 Chemistry Notes Form 1 Chemistry Notes Form 1 4 Chemistry Notes Form 1 Free Download Chemistry Notes Form 1 KLB Chemistry Notes Form 1 Pdf Chemistry Notes Form 1-4 Chemistry Notes Form 1-4(1) Chemistry Chemistry Notes Form 14 Chemistry Notes Form 2 Chemistry Notes Form 2 KLB Chemistry Notes Form 2 Pdf Chemistry Notes Form 2; Chemistry Notes Chemistry Notes Form 3 Chemistry Notes Form 3 KLB Chemistry Notes Form 3 Pdf Chemistry Notes Form 4 Chemistry Notes Form 4 Chapter 2 Chemistry Notes Form 4 KLB Chemistry Notes Form 4 Pdf Chemistry Notes Form 4-pdf Chemistry Notes Form Four Chemistry Notes Form Four KLB Chemistry Notes Form Four Pdf Chemistry Notes Form One Chemistry Notes Form One KLB Chemistry Notes Form One Pdf Chemistry Notes Form One to Form Four Chemistry Notes Form Three Chemistry Notes Form Three KLB Chemistry Notes Form Three Pdf Chemistry Notes Form Two Chemistry Notes Form Two KLB Chemistry Notes Form Two Pdf Chemistry Notes Form2 Chemistry Notes IGCSE Chemistry Notes Kenya Chemistry Notes on Agroforestry Chemistry Notes Pdf Chemistry Notes: Chemistry Objective Answer Chemistry Objective Answer 2018 Chemistry Objective Questions for Competitive Exams Chemistry Objective Questions for Competitive Exams Pdf Chemistry Oral Exam Questions Chemistry Paper 1 Chemistry Paper 1 2018 Marking Rules Chemistry Paper 1 Notes Chemistry Paper 1 Questions Chemistry Paper 1 Questions and Answers Chemistry Paper 1 Topics Chemistry Paper 1 With Answers Chemistry Paper 2 Chemistry Paper 2 2017 Chemistry Paper 2 2018 Marking Rules Chemistry Paper 2 Questions and Answers Chemistry Paper 2 Questions and Answers Pdf Chemistry Paper 2 Revision Chemistry Paper 2 Topics Chemistry Paper 2018 Chemistry Paper 3 2018 Marking Rules Chemistry Paper 3 Question and Answer Chemistry Paper 3 Question Paper 2014 KCSE Chemistry Paper 3 Question Paper 2015 KCSE Chemistry Paper 3 Question Paper 2016 KCSE Chemistry Paper 3 Question Paper 2017 KCSE Chemistry Paper 3 Question Paper 2018 KCSE Chemistry Paper 3 Questions and Answers Chemistry Paper One Questions and Answers Chemistry Paper One Topics Chemistry Paper Two Qestions With Answers Chemistry Paper1 Chemistry Paper2 Chemistry Paper3 Chemistry Paper4 Chemistry Past Papers Chemistry Past Papers 2017 Chemistry Past Papers a Level Chemistry Past Papers Form 1 Chemistry Past Papers Form 2 Chemistry Past Papers Form 3 Chemistry Past Papers O Level Chemistry Pdf Download Chemistry Pp1 KCSE 2016 Chemistry Practical Book Class 12 Pdf Chemistry Practical Exam Chemistry Practicals Form One Chemistry Practicals Questions and Answers Chemistry Practice Test 9th Grade Chemistry Practice Test Answers Chemistry Practice Test Questions and Answers Chemistry Practice Test Quizlet Chemistry Predicted Questions This Year KCSE Chemistry Preparation Notes Chemistry Pretest High School Pdf Chemistry Question and Answer With Explanation Chemistry Question and Answers 2019 Chemistry Question and Answers 2020 Chemistry Question and Answers 2021 Chemistry Question and Answers 2022 Chemistry Question and Answers Note Chemistry Questions Chemistry Questions and Answers Chemistry Questions and Answers for High School Chemistry Questions and Answers for High Schools Chemistry Questions and Answers for High Schools Pdf Chemistry Questions and Answers for Secondary Schools Chemistry Questions and Answers Form 1 Chemistry Questions and Answers Form 2 Chemistry Questions and Answers Form 3 Chemistry Questions and Answers Form 4 Chemistry Questions and Answers Multiple Choice Chemistry Questions and Answers Notes Chemistry Questions and Answers O Chemistry Questions and Answers Online Chemistry Questions and Answers Pdf Chemistry Questions and Answers Pdf for Class 12 Chemistry Questions and Answers Pdf for Competitive Exams Chemistry Questions and Answers-form 2 Chemistry Questions for High School Chemistry Questions for High School Students With Answers Chemistry Questions for Senior 1 Chemistry Questions for Senior 2 Chemistry Questions for Senior 3 Chemistry Questions for Senior 4 Chemistry Questions for Senior 5 Chemistry Questions for Senior 6 Chemistry Questions for Senior Five Chemistry Questions for Senior Four Chemistry Questions for Senior One Chemistry Questions for Senior Six Chemistry Questions for Senior Three Chemistry Questions for Senior Two Chemistry Questions Form One Chemistry Questions Multiple Choice Chemistry Questions Quizlet Chemistry Questions to Ask Your Teacher Chemistry Quetion and Answer Form Four Chemistry Quetion and Answer Form One Chemistry Quetion and Answer Form Three Chemistry Quetion and Answer Form Two Chemistry Quiz for Class 9 Chemistry Quiz for Class 9 Chemistry Chemistry Quiz Questions and Answers for Class 10 Chemistry Quiz Questions and Answers for Class 10 Pdf Chemistry Quiz Questions and Answers for Class 12 Chemistry Quiz Questions and Answers for Class 9 Chemistry Quiz Questions and Answers for Class 9 Pdf Chemistry Quiz Questions and Answers for High School Chemistry Quiz Questions and Answers Multiple Choice Chemistry Quiz Questions and Answers Pdf Chemistry Quiz Questions for Class 12 Chemistry Quiz Questions for College Students Chemistry Quiz With Answers Chemistry Quiz With Answers Pdf Chemistry Quizlet Chemistry Revision Chemistry Revision a Level Chemistry Revision Chemistry Notes Chemistry Chemistry Revision Exam Chemistry Revision Examination Chemistry Revision Form One Chemistry Revision Notes Chemistry Revision Notes Chemistry Chemistry Revision Notes Form 1 Chemistry Revision Notes Form 2 Chemistry Revision Notes Form 3 Chemistry Revision Notes Form 4 Chemistry Revision Notes IGCSE Chemistry Revision Paper One Chemistry Revision Questions Chemistry Revision Questions and Answers Chemistry Revision Questions and Answers Form 1 Chemistry Revision Questions and Answers Form 2 Chemistry Revision Questions and Answers Form 3 Chemistry Revision Questions and Answers Form 4 Chemistry Revision Questions and Answers Form Four Chemistry Revision Questions and Answers Form One Chemistry Revision Questions and Answers Form Three Chemistry Revision Questions and Answers Form Two Chemistry Revision Questions Form 1 Chemistry Revision Questions Form 2 Chemistry Revision Questions Form 3 Chemistry Revision Questions Form 4 Chemistry Revision Questions Form Four Chemistry Revision Questions Form One Chemistry Revision Questions Form Three Chemistry Revision Questions Form Two Chemistry Revision Quiz Chemistry Revision Test Chemistry Secondary School Revision Chemistry Simple Notes Chemistry Spm Notes Download Chemistry Spm Notes Pdf Chemistry Spm Questions Chemistry Study Form 2 Chemistry Study Guide Chemistry Study Guide Answer Key Chemistry Study Guide Answers Chemistry Study Guide Chemistry Questions and Answers Chemistry Study Guide Ib Chemistry Study Guide Pdf Chemistry Study Guides Chemistry Study Notes Chemistry Study Notes Materials Form 1 Pdf Chemistry Study Notes Materials Form 2 3 Pdf Chemistry Study Notes Materials Form 2 Pdf Chemistry Study Notes Materials Form 3 Pdf Chemistry Study Notes Materials Form 4 Pdf Chemistry Syllabus in Kenya Chemistry Syllabus Pdf Chemistry Test 1 Quizlet Chemistry Test Questions Chemistry Test Questions and Answers Chemistry Test Questions and Answers Pdf Chemistry Topic One Form Four Chemistry Topics Form One Chemistry Unit 1 Quiz Chemistry Vol 3 Chemistry | Revision Chemistry Chemistry,form 4 Chemistry.form Four.topic Three ChemistryExam Form Three ChemistryModule Form 5 ChemistryNotes ChemistryNotes for Class 11 Pdf ChemistryNotes for Class 12 Pdf ChemistryNotes Form 1 ChemistryNotes Form 1 Free Download ChemistryNotes Form 2 ChemistryNotes Form 3 ChemistryNotes Form 3 Pdf ChemistryNotes IGCSE ChemistryNotes Pdf ChemistryPast Papers ChemistryQuestions and Answers Pdf ChemistrySimple Notes ChemistrySpm Notes Download ChemistrySpm Notes Pdf ChemistrySpm Questions ChemistryStudy Guide Answers ChemistryStudy Guide Pdf ChemistryStudy Guides Blologytextpapers Bridge Chemistry Business Past KCSE Past Papers Business Studies Form 3 Notes Pdf Business Studies Form 4 Notes Pdf C R E Form One KLB C R E Form One Oli Topic C.r.e Form 1 Notes Kenya C.r.e Form 2 Notes Kenya C.r.e Form 3 Notes C.r.e Form 3 Notes Kenya C.r.e Form 3 Pdf C.r.e Form 4 Notes Kenya C.r.e Form One Notes Pdf C.r.e Notes Form 1 C.r.e Revision Notes C.r.e Short Notes Cambridge IGCSE Chemistry Cambridge IGCSE Chemistry 3rd Edition Cambridge IGCSE Chemistry 3rd Edition Plus Cd South Asia Edition Cambridge IGCSE Chemistry Answers Cambridge IGCSE Chemistry Coursebook Pdf Download Cambridge IGCSE Chemistry Practical Workbook Cambridge IGCSE Chemistry Revision Guide Pdf Cambridge IGCSE Chemistry Study and Revision Guide 2nd Edition Pdf Cambridge IGCSE Chemistry Study and Revision Guide Pdf Cambridge IGCSE Chemistry Workbook Free Download Cambridge IGCSE Chemistry Workbook Pdf Cambridge IGCSE® Chemistry Coursebook Caucasian Chalk Circle Essay Questions Chapter 1 Introduction to Chemistry Chapter 1 Introduction to Chemistry Studies Cie a Level Chemistry Notes 2016 Cie a Level Chemistry Notes Pdf Cie Past Papers Class 10 Chemistry Chapter 1 Mcqs Class 8 Chemistry Notes KCSE-kcse College Chemistry Notes College Chemistry Practice Test College Chemistry Quiz College Chemistry Quiz Chapter 1 College Chemistry Quizlet College Chemistry Study Guide College Chemistry Study Guide Pdf College Chemistry Test Questions and Answers College Chemistry Volume 3 Pdf College ChemistryNotes Complete Chemistry for Cambridge IGCSE Complete Chemistry for Cambridge IGCSE Revision Guide Pdf County Mocks 2017 Cse Past Papers Chemistry 2017 Dl Chemistry Form 3 Pdf Kusoma Download Chemistry Form 1 Download Chemistry Form 2 Download Chemistry Form 2 Notes Download Chemistry Form 3 Download Chemistry Form 3 Notes Download Chemistry Form 4 Download Chemistry Form Four Download Chemistry Form One Download Chemistry Form Three Download Chemistry Form Two Download Chemistry Notes Form 3 Download Chemistry Notes Form One Download ChemistryNotes Form 3 Download Form Three Chemistry Notes Download Free KCSE Past Papers Chemistry Download Free KCSE Past Papers From KNEC. Download KCSE Past Papers With Answers Download KCSE Revision Notes Download KLB Chemistry Book 2 Download KLB Chemistry Book 3 Download KLB Chemistry Book 4 Download Notes of Chemistry Downloads | Chemistry | Form Four Exams | Exams Downloads | Chemistry | Form One Exams | Exams Downloads | Chemistry | Form Three Exams | Exams Downloads | Chemistry | Form Two Exams | Exams Downloads | KCSE Papers and Marking Schemes | Dvance KCSE Past Papers Easy Chemistry Questions Edexcel a Level Chemistry B Edexcel a Level Chemistry Notes Pdf Edexcel a Level Chemistry Salters Nuffield Edexcel A2 Chemistry Notes Edexcel as Chemistry Revision Guide Pdf Edexcel Chemistry A2 Revision Notes Pdf Edexcel Chemistry Unit 2 Revision Notes Edexcel GCSE Chemistry Revision Guide Pdf Edexcel IGCSE Chemistry Past Papers Edexcel IGCSE Chemistry Revision Guide Free Pdf Download Edexcel IGCSE Chemistry Revision Guide Pdf Edexcel IGCSE Chemistry Revision Guide Pdf Download Electronics Form Four Notes Energy Questions Chemistry Bowl Essay Questions and Answers KCSE Chemistry Notes Essay Questions and Answers on Betrayal in the City Essay Questions Based on Betrayal in the City Evolving World Chemistry Book 1 Pdf Evolving World Chemistry Book 4 Notes Evolving World Chemistry Book Form 1 Evolving World-history Book 3 Exam Notes for Chemistry 101 Exams KCSE Chemistry Paper 1 Questions and Answers F3 Chemistry Test Paper Find Download KCSE Past Papers With Answers Find KCSE Chemistry Essay Questions and Answers Form 1 Chemistry Exam Form 1 Chemistry Notes Form 1 Chemistry Questions and Answers Form 1 Chemistry Questions and Answers Pdf Form 1 Chemistry Revision Notes Form 1 Chemistry Summurized Revision Pdf Form 1 Chemistry Syllabus Form 1 Chemistry Test Paper Pdf Form 1 Chemistry Topics Form 1 ChemistryNotes Form 1 ChemistryQuestions and Answers Form 1 ChemistryRevision Notes Form 1 ChemistrySyllabus Form 1 ChemistryTest Paper Pdf Form 1 Past Papers Form 1 Past Papers With Answers Form 1 Revision Papers Form 1 Subjects in Kenya Form 2 Chemistry Exam Form 2 Chemistry Exam Paper Form 2 Chemistry Exam Paper 2016 Form 2 Chemistry Exam Paper Free Download Form 2 Chemistry Exam Paper With Answer Form 2 Chemistry Final Year Exam Paper 2 Form 2 Chemistry Notes Form 2 Chemistry Notes and Revision Questions Form 2 Chemistry Notes Pdf Form 2 Chemistry Past Papers Form 2 Chemistry Questions Form 2 Chemistry Questions and Answers Form 2 Chemistry Questions and Answers > Form 2 Chemistry Questions and Answers Pdf Form 2 Chemistry Revision Notes Form 2 Chemistry Short Notes Form 2 Chemistry Syllabus Form 2 ChemistryExam Paper Form 2 ChemistryExam Paper Free Download Form 2 ChemistryExam Paper With Answer Form 2 ChemistryFinal Year Exam Paper 2 Form 2 ChemistryPast Papers Form 2 ChemistryRevision Notes Form 2 ChemistryShort Notes Form 2 ChemistrySyllabus Form 2 Revision Papers Form 2 Subjects in Kenya Form 3 Chemistry Book Form 3 Chemistry Exam Form 3 Chemistry Exam Paper Form 3 Chemistry Notes Form 3 Chemistry Past Papers Form 3 Chemistry Questions Form 3 Chemistry Questions and Answers Form 3 Chemistry Questions and Answers Pdf Form 3 Chemistry Revision Notes Form 3 Chemistry Syllabus Form 3 ChemistryExam Paper Form 3 ChemistryNotes Form 3 ChemistryPast Papers Form 3 ChemistryQuestions Form 3 ChemistryQuestions and Answers Pdf Form 3 ChemistryRevision Notes Form 3 ChemistrySyllabus Form 3 C.r.e Form 3 Notes of Chemistry Topic on Fish Form 3 Past Papers Form 3 Revision Papers Form 3 Subjects in Kenya Form 4 Chemistry Exam Form 4 Chemistry Notes Form 4 Chemistry Notes Pdf Form 4 Chemistry Questions and Answers Form 4 Chemistry Questions and Answers Pdf Form 4 Chemistry Revision Notes Form 4 Chemistry Syllabus Form 4 Chemistry Topics Form 4 ChemistryNotes Form 4 ChemistryRevision Notes Form 4 ChemistrySyllabus Form 4 ChemistryTopics Form 4 Exam Papers Form 4 Revision Papers Form 4 Subjects in Kenya Form 5 Chemistry Topics Form 5 ChemistryTopics Form Five Chemistry Notes Form Five ChemistryNotes Form Four Chemistry Book Form Four Chemistry Notes Form Four Chemistry Notes Pdf Form Four Chemistry Questions and Answers Form Four Chemistry Questions and Answers Pdf Form Four Chemistry Revision Questions Form Four Chemistry Syllabus Form Four Chemistry Topics Form Four ChemistryNotes Form Four ChemistryQuestions and Answers Form Four ChemistryQuestions and Answers Pdf Form Four ChemistryTopics Form Four Notes Form Four Revision Papers Form Four Subjects in Kenya Form One Chemistry Book Form One Chemistry Examination Form One Chemistry First Topic Form One Chemistry Lesson Plan Form One Chemistry Notes Pdf Form One Chemistry Past Papers Pdf Form One Chemistry Questions Form One Chemistry Questions and Answers Form One Chemistry Questions and Answers Pdf Form One Chemistry Revision Questions Form One Chemistry Short Notes Form One Chemistry Syllabus Form One Chemistry Topics Form One ChemistryExamination Form One ChemistryPast Papers Pdf Form One ChemistryQuestions and Answers Form One ChemistryQuestions and Answers Pdf Form One ChemistryTopics Form One Exams Form One Notes of Chemistry Form One Past Papers Form One Subjects in Kenya Form One Term One Chemistry Exam Form One Term One ChemistryExam Form Three Chemistry Book Form Three Chemistry Book Pdf Form Three Chemistry Notes Form Three Chemistry Notes Pdf Form Three Chemistry Questions and Answers Form Three Chemistry Questions and Answers Pdf Form Three Chemistry Revision Questions Form Three Chemistry Syllabus Form Three Chemistry Topics Form Three ChemistryNotes Form Three ChemistryNotes Pdf Form Three ChemistryQuestions and Answers Form Three ChemistryQuestions and Answers Pdf Form Three ChemistryTopics Form Three Subjects in Kenya Form Two Chemistry Book Form Two Chemistry Cat Form Two Chemistry Examination Form Two Chemistry Notes Form Two Chemistry Notes Pdf Form Two Chemistry Past Papers Form Two Chemistry Questions and Answers Form Two Chemistry Questions and Answers Pdf Form Two Chemistry Revision Questions Form Two Chemistry Syllabus Form Two Chemistry Topics Form Two ChemistryNotes Form Two ChemistryNotes Pdf Form Two ChemistryQuestions and Answers Form Two ChemistryQuestions and Answers Pdf Form Two ChemistrySyllabus Form Two ChemistryTopics Form Two Notes Form Two Subjects in Kenya Free a-level Chemistry Revision App | Pass Your Chemistry Exams Free Chemistry Form 1 Notes Free Chemistry Notes Form 1 Free Chemistry Notes Pdf Free ChemistryNotes Pdf Free College Chemistry Practice Test Free Form1,form2,form3 Past Papers Free KCSE Past Papers Free KCSE Mocks 2015 Free KCSE Past Papers 2014 Free KCSE Past Papers KCSE Past Free KCSE Past Papers Kenya, Free KCSE Past Papers With Answers Free KCSE Questions and Answers on Chemistry Free KCSE Revision Notes Free Marking Schemes Free Mocks Online KCSE Answers Past Exams Question Papers Free Revision Papers From Three Notes Topic One KLB Fun Chemistry Questions Funny Chemistry Questions Funny Chemistry Questions and Answers Funny Chemistry Questions to Ask Funny Chemistry Quotes GCSE Chemistry Exam Questions and Answers GCSE Chemistry Past Papers GCSE Chemistry Revision GCSE Chemistry Revision Notes GCSE Chemistry Revision Notes Pdf GCSE Chemistry Revision Notes Pdf 9-1 GCSE Chemistry Revision Questions and Answers GCSE Chemistry Textbook Pdf GCSE Chemistry Topics Pass My Exams: Easy Exam Revision Notes General Chemistry Notes Pdf General Chemistry Practice Test With Answers General Chemistry Quiz General Chemistry Quiz Pdf General Chemistry Test Questions and Answers General Chemistry Test Questions and Answers Pdf General Knowledge in Chemistry Human Body Good Chemistry Questions to Ask GRE Chemistry Practice Test GRE Chemistry Subject Test Pdf Handbook of Chemistry Pdf Free Download Hard Chemistry Questions Hard Chemistry Questions and Answers Hard Chemistry Questions to Ask Your Teacher Hard Chemistry Quiz Questions Hard Form 3 Chemistry Question High School Chemistry Final Exam Doc High School Chemistry Final Exam Pdf High School Chemistry Final Exam Questions High School Chemistry Final Exam Questions and Answers High School Chemistry Notes High School Chemistry Practice Test High School Chemistry Pretest With Answers High School Chemistry Questions and Answers Pdf High School Chemistry Study Guide High School Chemistry Test Questions and Answers Pdf High School ChemistryNotes High School ChemistryStudy Guide How to Answer KCSE Chemistry Question How to Motivate a Form 4 Student How to Motivate a KCSE Candidate How to Motivate a KCSE Student How to Pass Chemistry Questions & Answers Form 1&2 | Text Book How to Revise Chemistry How to Revise Effectively for KCSE How to Study Chemistry: 5 Study Techniques to Master Chemistry Hsc Chemistry 2018 Hsc Chemistry 2019 Https://www.knec.ac.ke/ Www.knec-portal.ac.ke/ KNEC Portal: Ial Chemistry Notes Ib Chemistry Cold War Notes Ib Chemistry Notes Ib Chemistry Notes Pdf Ib Chemistry of the Americas Notes Ib Chemistry of the Americas Study Guide Ib Chemistry Paper 2 Study Guide Ib Chemistry Question Bank by Topic Ib Chemistry Study Guide Pdf Ict Notes Form 1 IGCSE Chemistry Alternative to Practical Revision IGCSE Chemistry Alternative to Practical Revision Notes IGCSE Chemistry Book IGCSE Chemistry Book Pdf Download IGCSE Chemistry Notes IGCSE Chemistry Notes 2017 Pdf IGCSE Chemistry Notes Edexcel IGCSE Chemistry Paper 2 Notes IGCSE Chemistry Paper 6 Notes IGCSE Chemistry Past Papers IGCSE Chemistry Past Papers 2014 IGCSE Chemistry Past Papers 2017 IGCSE Chemistry Pdf IGCSE Chemistry Pre Release Material 2018 IGCSE Chemistry Resources IGCSE Chemistry Revision Guide IGCSE Chemistry Revision Guide Free Download IGCSE Chemistry Revision Guide Pdf Download IGCSE Chemistry Revision Notes Pdf IGCSE Chemistry Revision Worksheets IGCSE Chemistry Workbook Pdf IGCSE Chemistry Znotes IGCSE ChemistryPast Papers IGCSE Notes Chemistry Importance of Agroforestry Inorganic Chemistry Multiple Choice Questions With Answers Pdf Inorganic Chemistry Questions and Answers Pdf Interesting Chemistry Questions Interesting Chemistry Questions and Answers Interesting Questions to Ask About Chemistry Intro to Chemistry Quiz Introduction of Chemistry Form One Introduction to Chemistry Introduction to Chemistry Notes Introduction to Chemistry Pdf Introduction to ChemistryNotes Is Agroforestry Sustainable? K.c.s.e Answers Chemistry Paper One 2018 K.c.s.e Chemistry 2017 K.c.s.e Chemistry 2018 K.c.s.e Chemistry Paper 1 2017 K.c.s.e Mocks 2018 K.c.s.e Papers 2015 K.c.s.e Papers 2016 K.c.s.e Past Papers 2014 K.c.s.e.Chemistry Paper 2 Year 2018 K.c.s.e.results 2018 for Busia County K.l.b Chemistry Form 3 K.l.b Chemistry Notes K.l.b ChemistryNotes Kasneb Past Papers for Colleges Chemistry Past Papers KCSE 2010 Marking Scheme KCSE 2010 Past Papers KCSE 2011 Chemistry Paper 1 KCSE 2011 Marking Scheme KCSE 2012 Chemistry Paper 2 Marking Scheme KCSE 2012 Marking Schemes KCSE 2013 Chemistry Paper 1 KCSE 2013 Marking Scheme KCSE 2013 Marking Scheme Pdf KCSE 2014 KCSE 2015 Chemistry Paper 2 KCSE 2015 Chemistry Paper 3 KCSE 2015 Marking Scheme KCSE 2015 Past Papers KCSE 2016 Chemistry Paper 1 KCSE 2016 Chemistry Paper 2 KCSE 2017 Chemistry Paper 1 KCSE 2017 Chemistry Paper 2 KCSE 2017 Hostory Papers With Answers.com KCSE 2017 Marking Scheme KCSE 2017 Papers KCSE 2017 Papers and Marking Scheme KCSE 2017 Papers Pdf KCSE 2017 Past Papers KCSE 2017 Prediction Pdf KCSE 2018 Chemistry and Answers KCSE 2018 Chemistry Prediction KCSE 2018 Leakage KCSE 2018 Marking Scheme KCSE 2018 Papers KCSE 2018 Prediction Pdf KCSE 2018 Predictions KCSE 2018 Questions KCSE 2018 Questions and Answers KCSE 2019 Leakage Chemistry KCSE 2019 Marking Scheme KCSE 2019 Questions KCSE 2019 Questions and Answers KCSE 2020 Questions KCSE 2020 Questions and Answers KCSE Answers KCSE Answers Past Exams Question Papers Downloads | KCSE Chemistry 2011 KCSE Chemistry 2016 KCSE Chemistry Diagramsbiology Revision Tips KCSE Chemistry Essay Questions and Answers KCSE Chemistry Essay Questions and Answers Pdf KCSE Chemistry Essays KCSE Chemistry Essays Pdf KCSE Chemistry Marking Schemes KCSE Chemistry Notes KCSE Chemistry Notes Pdf KCSE Chemistry Notes, Syllabus, Questions, Answers KCSE Chemistry Paper 1 KCSE Chemistry Paper 1 2011 KCSE Chemistry Paper 1 2012 KCSE Chemistry Paper 1 2013 KCSE Chemistry Paper 1 2015 KCSE Chemistry Paper 1 2016 KCSE Chemistry Paper 1 2017 KCSE Chemistry Paper 1 2017 Pdf KCSE Chemistry Paper 1 Questions and Answers KCSE Chemistry Paper 2 KCSE Chemistry Paper 2 2012 KCSE Chemistry Paper 2 2012 KCSE Chemistry Paper 2 2015 KCSE Chemistry Paper 2 2013 KCSE Chemistry Paper 2 2014 KCSE Chemistry Paper 2 2015 KCSE Chemistry Paper 2 2016 KCSE Chemistry Paper 2 2017 KCSE Chemistry Paper 3 KCSE Chemistry Paper 3 2012 KCSE Chemistry Paper 3 2016 KCSE Chemistry Paper 3 2017 KCSE Chemistry Paper 3 Past Papers KCSE Chemistry Past Papers KCSE Chemistry Past Papers and Answers KCSE Chemistry Past Papers Pdf KCSE Chemistry Practical KCSE Chemistry Practical 2015 KCSE Chemistry Practical 2016 KCSE Chemistry Practical Past Papers KCSE Chemistry Practicals KCSE Chemistry Practicals KCSE Chemistry Paper 1 KCSE Chemistry Question and Answer KCSE Chemistry Questions and Answers KCSE Chemistry Questions and Answers Ap Chemistry KCSE Chemistry Revision KCSE Chemistry Revision Notes KCSE Chemistry Revision Papers KCSE Chemistry Revision Questions KCSE Chemistry Revision Questions and Answers KCSE Chemistry Syllabus KCSE ChemistryNotes KCSE ChemistryPaper 1 KCSE ChemistryPaper 2 KCSE ChemistryPaper 2 Pdf KCSE ChemistrySyllabus KCSE Business Paper 1 2016 KCSE Business Past Papers KCSE Business Studies Past Papers KCSE Essay Questions in Betrayal in the City KCSE Essays KCSE Exam Papers 2018 KCSE Exam Papers Answers KCSE Form 1 Chemistry Revision KCSE Form 2 Chemistry Revision KCSE Form 3 Chemistry Revision KCSE Form 4 Chemistry Revision KCSE Form Four Chemistry Revision KCSE Form One Chemistry Revision KCSE Form Three Chemistry Revision KCSE Form Two Chemistry Revision KCSE KCSE Past Papers KNEC KCSE Leakage KCSE Leakage Chemistry KCSE Made Familiar Chemistry KCSE Made Familiar Chemistry Pdf KCSE Marking Scheme 2016 KCSE Marking Schemes KCSE Marking Schemes 2017 KCSE Marking Schemes Pdf KCSE Mock Exams KCSE Mock Papers 2015 KCSE Mock Papers 2017 KCSE Mock Papers 2018 KCSE Mock Papers Pdf KCSE Mock Papers Pdf 2018 KCSE Mock Papers Pdf KCSE Past Papers KCSE Mocks 2017 KCSE Mocks 2018 KCSE Notes KCSE Online Notes KCSE Online Past Papers KCSE Online Registration KCSE Papers 2015 KCSE Papers and Marking Schemes | Exams KCSE Past Papers KCSE Past Papers 2007 KCSE Past Papers 2009 KCSE Past Papers 2010 KCSE Past Papers 2011 KCSE Past Papers 2011 Pdf KCSE Past Papers 2012 KCSE Past Papers 2013 KCSE Past Papers 2013knec KCSE Past Papers 2014 KCSE Past Papers 2014 Pdf KCSE Past Papers 2015 KCSE Past Papers 2015 Marking Schemes KCSE Past Papers 2015 Pdf KCSE Past Papers 2016 KCSE Past Papers 2016 Pdf KCSE Past Papers 2017 KCSE Past Papers 2017 Pdf KCSE Past Papers 2018 KCSE Past Papers Chemistry KCSE Past Papers Chemistry and Answers KCSE Past Papers Chemistry Pdf KCSE Past Papers Chemistry With Answers KCSE Past Papers Chemistryand Answers KCSE Past Papers Business Studies and Answers KCSE Past Papers KCSE and Answers KCSE Past Papers KCSE and Answers Free Mocks Online KCSE Past Papers Marking Scheme KCSE Past Papers Pdf Download KCSE Past Papers Pdf Download KCSE 2013 KCSE Past Papers With Answers KCSE Past Papers Woodwork and Answers KCSE Prediction 2017 KCSE Prediction 2018 KCSE Prediction 2018 Pdf KCSE Prediction Papers 2018 KCSE Prediction Questions KCSE Prediction Questions 2018 KCSE Prediction Questions and Answers KCSE Questions KCSE Questions and Answers KCSE Questions and Answers. KCSE Questions on Chemistry KCSE Results, Online Registration, KCSE Result Slip. KCSE Revision KCSE Revision Notes KCSE Revision Notes Chemistry KCSE Revision Notes Pdf KCSE Revision Papers KCSE Revision Papers 2014 KCSE Revision Papers With Answers KCSE Revision Question for Chemistry KCSE Revision Questions KCSE Revision Questions and Answers KCSE Revision | Secondary School | Text Books | Text Book Centre KCSE Syllabus Pdf KCSE Trial 2017 KCSE Trial Exams 2017 Kenya Secondary School Chemistry Syllabus Kenya Secondary School Chemistry Syllabus Pdf Kenya Secondary School ChemistrySyllabus Pdf Kenya Secondary School Syllabus Pdf Kenya-kcse-christian Religious Education Syllabus Kenyaplex KCSE Past Papers Kenyaplex Past Papers for Secondary KLB Chemistry Book 1 Download KLB Chemistry Book 1 Notes KLB Chemistry Book 1 Pdf KLB Chemistry Book 2 KLB Chemistry Book 2 Notes KLB Chemistry Book 2 Notes Pdf KLB Chemistry Book 2 Pdf KLB Chemistry Book 3 Notes KLB Chemistry Book 3 Pdf KLB Chemistry Book 3 Pdf Download KLB Chemistry Book 4 Notes KLB Chemistry Book 4 Pdf KLB Chemistry Book 4 Pdf Download KLB Chemistry Book 4 Topics KLB Chemistry Book One KLB Chemistry Form 1 KLB Chemistry Form 1 Notes KLB Chemistry Form 1 Pdf KLB Chemistry Form 2 KLB Chemistry Form 2 Book KLB Chemistry Form 2 Notes KLB Chemistry Form 2 Pdf KLB Chemistry Form 2 Pdf Download KLB Chemistry Form 2 Schemes of Work KLB Chemistry Form 3 KLB Chemistry Form 3 Notes KLB Chemistry Form 3 Notes Pdf KLB Chemistry Form 3 Pdf KLB Chemistry Form 3 Pdf Download KLB Chemistry Form 4 KLB Chemistry Form 4 Notes KLB Chemistry Form 4 Pdf KLB Chemistry Form Four KLB Chemistry Form Four Notes KLB Chemistry Form One KLB Chemistry Form One Notes KLB Chemistry Form Three KLB Chemistry Form Three Notes KLB Chemistry Form Two KLB Chemistry Form Two Notes KLB Chemistry Notes KLB Chemistry Notes Form 4 KLB Chemistry Pdf KLB ChemistryNotes KLB ChemistryNotes Form 4 KLB ChemistryPdf KNEC Chemistry Syllabus KNEC Examiners Portal KNEC Website KNEC Ict Past Papers KNEC Past Papers for Colleges KNEC Past Papers Free Download KNEC Past Papers Free Downloads KNEC Past Papers Pdf KNEC Portal Confirmation KNEC Portal KCSE Results KNEC Portal KNEC Past Papers for Colleges Kasneb Past Papers KNEC Revision Papers KNEC Technical Exams Past Papers Kusoma Chemistry Notes Kusoma Chemistry Notes Pdf Kusoma Notes Chemistry Kusoma.co.ke Kusoma.com Past Papers Learner Guide for Cambridge IGCSE Chemistry Longhorn Chemistry Book 3 Pdf Made Familiar Chemistry Made Familiar Chemistry Pdf Made Familiar Chemistry Questions Maktaba Tetea Notes Marking Scheme KCSE Chemistry Past Papers Math Form2 Note Mcqs About Gaseous Exchange Middle School Chemistry Bowl Chemistry Questions Mock Past Papers 2017 Mock Past Papers With Answers Mokasa Mock 2017 More Than 1800 Chemistry Questions and Answers to Help You Study Multiple Choice Questions on Chemistry Necta Chemistry Past Papers Necta Chemistry Practicals Necta ChemistryPast Papers Necta ChemistryPracticals Necta Form Four Past Papers Necta Past Papers Form 4 Necta Past Papers Form 4 2016 Necta Past Papers Form Six Necta Past Papers Form Two Necta Questions and Answers Necta Review Questions Notes Chemistry Form 1 Notes Chemistry Form 2 Notes Chemistry Form 3 Notes Chemistry Form 3 Notes Pdf Notes Chemistry Form 3 Syllabus Notes Chemistry Form 4 Syllabus Notes on Chemistry Studies Notes Za Chemistry 4m 2 Notes Za Chemistry Form One Notes Za Chemistry Form Three O Level Chemistry Practical Experiments O Level Chemistry Questions and Answers Pdf Orm Three Chemistry Notes Page Navigation Papacambridge Chemistry IGCSE Papers KNEC KCSE Online Past Papers KNEC KCSE Results Past Papers Past KCSE Papers Past Paper Questions by Topic Chemistry Past Papers 2014 Past Papers in Kenya Pdf Chemistry Form 3 Pdf Chemistry Notes Pdf Chemistry Notes Form 1 Pdf Chemistry Notes Form 2 Pdf Chemistry Notes Form 3 Pdf Chemistry Notes Form 4 Pdf Chemistry Notes Form Four Pdf Chemistry Notes Form One Pdf Chemistry Notes Form Three Pdf Chemistry Notes Form Two Pdf Form 1 Chemistry Questions and Answers Pdf Form 2 Chemistry Questions and Answers Pdf Form 3 Chemistry Questions and Answers Pdf Form 4 Chemistry Questions and Answers Pdf Form Four Chemistry Questions and Answers Pdf Form One Chemistry Questions and Answers Pdf Form Three Chemistry Questions and Answers Pdf Form Two Chemistry Questions and Answers Pdf Free KCSE Past Papers and Marking Schemes Pdf" Revision Questions Chemistry Form 1 Practical Chemistry Experiments Pdf Practical Chemistry Question and Answer Pdf Pre Mocks 2018 Preliminary Chemistry Primary and Secondary Tillage Implements Ppt Pte KNEC Past Papers Questions and Answers Pdf Chemistry Form 1 Questions and Answers Pdf Chemistry Form 2 Questions and Answers Pdf Chemistry Form 3 Questions and Answers Pdf Chemistry Form 4 Questions and Answers Pdf Chemistry Form Four Questions and Answers Pdf Chemistry Form One Questions and Answers Pdf Chemistry Form Three Questions and Answers Pdf Chemistry Form Two Questions Based to Introduction to Chemistry Questions on Gaseous Exchange in Humans Questions on Introduction to Chemistry Questions to Ask in Chemistry Class Questions to Confuse Your Chemistry Teacher Quizlet Chemistry Test Quizlet Test Questions Qustions in Chemistry and Answers Revision Revision Chemistry Notes and Questions? Revision Quiz for Chemistry for Form Three S.1 Chemistry Questions S.2 Chemistry Questions S.3 Chemistry Questions S.4 Chemistry Questions Sample Essays on Betrayal in the City School Chemistry Notes Secondary Chemistry Notes Secondary Chemistry Notes Pdf Secondary ChemistryNotes Pdf Senior 1 Chemistry Notes Senior 2 Chemistry Notes Senior 3 Chemistry Notes Senior 4 Chemistry Notes Senior 5 Chemistry Notes Senior 6 Chemistry Notes Senior Five Chemistry Notes Senior Four Chemistry Notes Senior One Chemistry Notes Senior Six Chemistry Notes Senior Three Chemistry Notes Senior Two Chemistry Notes Simple Scientific Questions Smart Questions to Ask a Chemistry Teacher Snab Chemistry Revision Notes Southwest Mock Paper 2 2016 Chemistry Only Spm Chemistry Revision Notes Spm Notes Success Chemistry Spm Pdf Success ChemistrySpm Pdf Summary of Chemistry Form 3 Tahossa Past Papers To Motivate a Form 4 KCSE Student To Motivate a Form 4 Student Topical Revision Material Tricky Chemistry Questions and Answers Tricky Chemistry Questions for Adults Tricky Chemistry Questions With Answers Tricky Chemistry Quiz Questions Two Chemistry Revision Questions University Chemistry Volume 3 Openstax University Chemistry Volume 3 Pdf University Chemistry Volume 4 Pdf Ur Revision Guide IGCSE Chemistry What Are the Types of Gametes Working of Excretory System Www.Chemistry Form One Notes.com Www.Chemistry From One KLB.com Www.form 1 Chemistry.com Www.form 2 Chemistry.com Www.form 3 Chemistry.com Www.form 4 Chemistry.com Www.form Four Chemistry.com Www.form One Chemistry.com Www.form Three Chemistry.com Www.form Two Chemistry.com Www.kusoma Notes Www.kusoma Revision Materials Www.kusoma.co.ke Chemistry Notes Xtremepapers IGCSE Chemistry Year 11 Chemistry Z Notes Chemistry IGCSE Znotes as Chemistry Acids and Bases Chem Revision Questions Chemistry Notes Combine Form 1, 2, 3 and 4 Chemistry Notes Form 3 A Level Chemistry Questions and Answers Pdf All Chemistry Questions and Answers Pdf,ppt Chemistry Exam Questions and Answers Pdf Chemistry Multiple Choice Questions and Answers Pdf Chemistry Questions and Answers for High Schools Pdf Chemistry Notes Form 1-4 Pdf Free High School Notes Kenya Free Kcse Revision Notes General Chemistry Test Questions and Answers Pdf High School Chemistry Multiple Choice Questions and Answers Pdf Kcse Chemistry Revision Notes Pdf Kcse Revision Books Pdf Kcse Revision Notes Pdf Kenya Secondary School Notes Pdf Notes of Form 123 and 4 All Subject Chemistry Notes Form 1 Free Download Advice to KCSE Candidates Best Revision Books for KCSE How to Pass an Exam Successfully How to Pass KCSE 2018 How to Pass KCSE 2019 How to Pass KCSE Chemistry Paper How to Pass KCSE Chemistry How to Pass KCSE Chemistry How to Pass KCSE 2019 K.c.s.e Chemistry Paper 1 2017 KCSE 2019 Prediction KCSE Prediction 2019 KCSE Revision Tips KCSE Chemistry Paper 1 2018 KCSE Chemistry Paper 2 2018 KCSE Chemistry Past Papers Pdf KCSE Past Papers 2012 KCSE Past Papers 2017 Pdf KCSE Past Papers 2018 KCSE Past Papers of Chemistry Pp2 KCSE Chemistry Past Papers Pdf Chemistry KCSE Papers With Their Marking Schemes Chemistry Paper 1 and Answers KCSE 2017 Papers and Marking Scheme KCSE 2019 Papers and Marking Scheme KCSE Chemistry Paper 1 2018 KCSE Chemistry Paper 1 2019 KCSE Chemistry Paper 2 2017 KCSE Chemistry Paper 2 2018 KCSE Chemistry Paper 2 2019 KCSE Chemistry Past Papers and Answers KCSE Marking Schemes Pdf KCSE Past Papers 2015 Marking Schemes KCSE Past Papers 2019 Marking Schemes Chemistry KCSE Papers With Their Marking Schemes Chemistry Paper 1 and Answers KCSE 2017 Papers and Marking Scheme KCSE 2019 Papers and Marking Scheme KCSE Chemistry Paper 1 2018 KCSE Chemistry Paper 1 2019 KCSE Chemistry Paper 1 2019 Past Papers KCSE Chemistry Paper 2 2017 KCSE Chemistry Paper 2 2018 KCSE Chemistry Paper 2 2019 KCSE Chemistry Paper 2 2019 Past Papers KCSE Chemistry Paper 3 2019 Past Papers KCSE Chemistry Past Papers and Answers KCSE Marking Schemes Pdf KCSE Past Papers 2015 Marking Schemes KCSE Past Papers 2019 Marking Schemes KCSE Past Papers Chemistry Paper 1 2019 KCSE Past Papers Chemistry Paper 2 2019 KCSE Past Papers Chemistry Paper 3 2019 Past Papers KCSE Chemistry Paper 1 2019 Past Papers KCSE Chemistry Paper 2 2019 Past Papers KCSE Chemistry Paper 3 2019 Chemistry Form 1 Questions and Answers Chemistry Paper 1 2018 Chemistry Paper 2 2018 Chemistry Paper 1 2019 Chemistry Paper 2 2019 Chemistry Paper 1 Notes Chemistry Paper 1 Questions and Answers Pdf Chemistry Paper 2 Questions and Answers Chemistry Questions and Answers Form 2 KCSE Chemistry Paper 1 2018 KCSE Chemistry Paper 2 2018 KCSE Chemistry Paper 1 2017 KCSE Chemistry Paper 2 2017 KCSE Chemistry Paper 1 2019 KCSE Chemistry Paper 2 2019 Most Tested KCSE Chemistry Questions 4m1 Notes Viusasa 4m2 Notes Viusasa 4m3 Notes Viusasa 4m4 Notes Viusasa Download on Viusasa - Download Now for Free Elimu - Viusasa Elimu Library | Notes, Exams, Lesson Plans, Schemes Elimu Online High School Notes - Revision Materials for Kenyan Schools Https //www.viusasa.com/elimu Kenya Notes Viusasa Elimu Form 1 Notes Viusasa Elimu Form 2 Notes Viusasa Elimu Form 3 Notes Viusasa Elimu Form 4 Notes Viusasa Elimu Form Four Notes Viusasa Elimu Form One Notes Viusasa Elimu Form Three Notes Viusasa Elimu Form Two Viusasa Viusasa Education Viusasa Elimu Viusasa Elimu Class 6 Viusasa Elimu Form 1 Viusasa Elimu Form 1 Notes Viusasa Elimu Form 2 Viusasa Elimu Form 2 Notes Viusasa Elimu Form 3 Viusasa Elimu Form 3 Notes Viusasa Elimu Form 4 Viusasa Elimu Form 4 Notes Viusasa Elimu Form Four Viusasa Elimu Form Four Notes Viusasa Elimu Form One Viusasa Elimu Form One Notes Viusasa Elimu Form Three Viusasa Elimu Form Three Notes Viusasa Elimu Form Two Viusasa Elimu Form Two Notes Viusasa High School Notes - Revision Materials for Kenyan Schools Viusasa Notes Chemistry Mock Papers Chemistry Paper 1 2019 Chemistry Paper 1 Notes Chemistry Paper 1 Questions and Answers Chemistry Paper 1 Questions and Answers Pdf Chemistry Paper 2 Form 3 Chemistry Paper 2 Notes Chemistry Paper 2 Questions and Answers Pdf Chemistry Past Papers Chemistry Past Papers Pdf Chemistry Questions and Answers Pdf Chemistry Revision Questions and Answers Pdf Chemistry Revision Questions Pdf Common Exam Questions in Chemistry Paper 1 Common Exam Questions in Chemistry Paper 2 Common Test Questions in Chemistry Paper 1 Common Tested Questions in Chemistry Paper 1 Commonly Tested Questions in Chemistry Paper 1 K.c.s.e.Chemistry Questions and Answers KCSE 2019 Chemistry Paper 1 Marking Scheme KCSE 2019 Chemistry Paper 2 KCSE 2019 Chemistry Paper 2 Marking Scheme KCSE 2020 Prediction Questions and Answers KCSE Chemistry Paper 1 2019 KCSE Chemistry Paper 2 2016 KCSE Chemistry Paper 2 2017 KCSE Chemistry Paper 2 2018 KCSE Chemistry Paper 2 2019 KCSE Chemistry Questions and Answers Most Tested Questions in Chemistry Paper 1 Most Tested Questions in Chemistry Paper 2 Mostly Tested Questions in Chemistry Paper 1 Mostly Tested Questions in Chemistry Paper 2 Chemistry Form 4 Chemistry Revision Questions Form 1 Combined Science Notes Form 1 Form 4 Notes High Flyer Series Chemistry Form 1-4 Kcse Past Papers Chemistry With Answers Chemistry Notes Download Secondary Chemistry Notes Pdf Water and Hydrogen Form 1 Notes High Flyer Series KCSE Revision in Chemistry High Flyer Series KCSE Revision Chemistry Form 1-4 Revised A Level Chemistry Notes Uganda Pdf Download Buddo Junior School Holiday Work Chemistry Notes for a Level Pdf Chemistry Notes Form 1 Chemistry Notes Form 3 the Mole Chemistry Notes Form 4 Chemistry Notes O Level Chemistry Notes O Level Uganda Chemistry Notes O Level Uganda Pdf Chemistry Notes Pdf Download Chemistry Notes Form 3 Chemistry Notes Form 3 Chemistry Questions and Answers Form Three Chemistry Syllabus Pdf Gayaza High School Chemistry Notes Gayaza High School Chemistry Notes Pdf Gayaza High School Chemistry Past Papers Gayaza High School Chemistry Past Papers Answers Gayaza High School Chemistry Past Papers Questions and Answers Gayaza High School E Learning Gayaza High School Elearning Platform Gayaza High School Examinations Gayaza High School Holiday Work Gayaza High School Notes Gayaza High School Notes 2021 Gayaza High School Notes 2022 Gayaza High School Notes 2023 Gayaza High School Notes Pdf Gayaza Junior School E Learning Platform Gayaza Junior School Holiday Work Gayaza O Level Chemistry Notes Home › Gayaza High School | Elearning Platform Kabojja Junior School Holiday Work Pdf Klb Chemistry Book 3 Klb Chemistry Form 3 Teachers Guide Klb Chemistry Notes List of Ugandan E-learning Platforms for Students Mirembe Junior School Holiday Work Ntare School Past Papers O Level Chemistry Notes Free Download O Level Chemistry Notes in Uganda O Level Chemistry Notes Pdf O Level Chemistry Notes Uganda Pdf O Level Chemistry Notes Uganda Pdf Download O Level General - Home › Gayaza High School | Elearning S.1 Chemistry Notes | Standard High School Zzana S.3 Chemistry Notes S.4 Chemistry 2 Notes | Standard High School Zzana Seeta High School Elearning Seeta High School Holiday Work Seeta High School Notes Seeta High School Notes Pdf Seeta High School Past Papers Seeta High School Past Papers Pdf Senior 1 Chemistry Notes Senior 1 Chemistry Notes in Uganda Senior 1 Chemistry Notes Uganda Senior 1 Chemistry Questions Senior 1 Exams Senior 1 Work Senior 1 Work 2020 Senior 1 Work 2020 Uganda Senior 2 Chemistry Notes Senior 2 Chemistry Notes in Uganda Senior 2 Chemistry Notes Uganda Senior 2 Chemistry Questions Senior 2 Exams Senior 2 Work Senior 2 Work 2020 Senior 2 Work 2020 Uganda Senior 3 Chemistry Notes Senior 3 Chemistry Notes in Uganda Senior 3 Chemistry Notes Uganda Senior 3 Chemistry Questions Senior 3 Exams Senior 3 Work Senior 3 Work 2020 Senior 3 Work 2020 Uganda Senior 4 Chemistry Notes Senior 4 Chemistry Notes in Uganda Senior 4 Chemistry Notes Uganda Senior 4 Chemistry Questions Senior 4 Exams Senior 4 Work Senior 4 Work 2020 Senior 4 Work 2020 Uganda Senior Four Chemistry Notes Senior Four Chemistry Notes in Uganda Senior Four Chemistry Notes Uganda Senior Four Chemistry Questions Senior Four Exams Senior Four Work Senior Four Work 2020 Senior Four Work 2020 Uganda Senior One Chemistry Notes Senior One Chemistry Notes in Uganda Senior One Chemistry Notes Uganda Senior One Chemistry Questions Senior One Exams Senior One Work Senior One Work 2020 Senior One Work 2020 Uganda Senior Three Chemistry Notes Senior Three Chemistry Notes in Uganda Senior Three Chemistry Notes Uganda Senior Three Chemistry Questions Senior Three Exams Senior Three Work Senior Three Work 2020 Senior Three Work 2020 Uganda Senior Two Chemistry Notes Senior Two Chemistry Notes in Uganda Senior Two Chemistry Notes Pdf Senior Two Chemistry Notes Uganda Senior Two Chemistry Questions Senior Two Exams Senior Two Work Senior Two Work 2020 Senior Two Work 2020 Uganda St Mary's Kitende E Learning Standard High School Notes Standard High School Zana a Level Notes Standard High School Zana Com Notes Standard High School Zana E Learning Standard High School Zana E Learning Platform Standard High School Zana E-learning Platform Standard High School Zana Notes for Senior Two Standard High School Zana Notes Pdf Standard High School Zana Website Standard High Zana Notes Uace Chemistry Notes Uace Chemistry Notes Pdf Uce Chemistry Notes Pdf Uce Chemistry Notes Pdf Download Uce Past Papers Uganda Secondary Schools E-learning Platform Uneb Marking Guides Pdf Uneb Past Papers and Answers Pdf Air and Combustion Form 1 Notes Chemistry Form 1 Notes Pdf Free Download Chemistry Form One Questions and Answers Chemistry Notes Form 1 Easy Elimu Chemistry Notes Form 1-4 Pdf Chemistry Notes Form 1-4 Pdf Download Free High School Chemistry Notes Pdf Klb Chemistry Form 1 Notes Pdf Download Notes of Form One Chemistry Laboratory and Drawings Chemistry Form 1 Questions and Answers Pdf Download Chemistry Notes Form 1-4 Chemistry Form 1 Questions and Answers Pdf Form 1 Chemistry Topical Questions Form 1 Chemistry Exam Paper With Answer Pdf Form 1 Exams 2020 Form 1 Chemistry Past Papers Form 1 Chemistry Revision Papers With Answers Sample Chemistry Test for Form One Exams Ntare School Past Papers Seeta High School Notes Pdf Seeta High School Past Papers Seeta High School Past Papers Pdf St Mary's Kitende Past Papers Uce Chemistry Notes Pdf Uce Past Papers Uneb Marking Guides Pdf Uneb Past Papers and Answers Pdf Chemistry Book 3 Download Chemistry Form 3 Questions and Answers Chemistry Form 3 Syllabus Chemistry Form 3 Topics Chemistry Notes Form 3 Chemistry Notes Form 3 Chemistry Notes Form 3 Chemistry Notes Form Three Chemistry Notes Pdf Download Form 3 Chemistry Exam Form 3 Chemistry Notes on Pdf Revision Chemistry Revision a Level Chemistry Revision Notes - Chemistry S.1-Chemistry-notes S.1-Chemistry-notes Uganda S.2-Chemistry-notes S.2-Chemistry-notes Uganda S.3-Chemistry-notes S.3-Chemistry-notes Uganda S.4-Chemistry-notes S.4-Chemistry-notes Uganda S.5-Chemistry-notes S.5-Chemistry-notes Uganda S.6-Chemistry-notes S.6-Chemistry-notes Uganda S1 Chemistry Notes Term 1 S1 Chemistry Notes Term 2 S1 Chemistry Notes Term 3 S2 Chemistry Notes Term 1 S2 Chemistry Notes Term 2 S2 Chemistry Notes Term 3 S3 Chemistry Notes Term 1 S3 Chemistry Notes Term 2 S3 Chemistry Notes Term 3 S4 Chemistry Notes Term 1 S4 Chemistry Notes Term 2 S4 Chemistry Notes Term 3 Senior 3 Chemistry Notes Senior 3 Chemistry Notes Uganda Senior Three Chemistry Notes Senior Three Chemistry Notes Uganda Chemistry Form 1 the Cell Chemistry Form One Notes Free – Education News Chemistry Notes Form 1-4 Chemistry Questions and Answers Form 1 - Chemistry Form One Chemistry Questions and Answers Form 2 - Chemistry Form Two Chemistry Questions and Answers Form 3 - Chemistry Form Three Chemistry Questions and Answers Form 4 - Chemistry Form Four Chemistry Questions and Answers Senior 1 - Chemistry Senior One Chemistry Questions and Answers Senior 2 - Chemistry Senior Two Chemistry Questions and Answers Senior 3 - Chemistry Senior Three Chemistry Questions and Answers Senior 4 - Chemistry Senior Four Chemistry Topic by Topic Questions and Answers Combined Science Notes Form 1 Form 1 Chemistry Revision Questions and Answers Form 1 Chemistry Topical Questions Form 1 Revision Papers With Answers Form 2 Chemistry Revision Questions and Answers Form 3 Chemistry Revision Questions and Answers Form Four Chemistry Revision Questions and Answers Form One Chemistry Revision Questions and Answers Form Three Chemistry Revision Questions and Answers Form Two Chemistry Revision Questions and Answers Free Chemistry Form 1 Notes Free Chemistry Notes Introduction to Chemistry Form One Kcse Past Papers: Chemistry Form 1 Kcse Past Papers: Chemistry Form 1 Topical Questions Kcse Past Papers: Chemistry Form 1 Topical Questions and Answers Most Tested Areas in Chemistry Kcse Most Tested Areas in Chemistry Kcse Exams Most Tested Areas in Chemistry Uneb Most Tested Areas in Form 1 in Chemistry Most Tested Areas in Form 2 in Chemistry Most Tested Areas in Form 3 in Chemistry Most Tested Areas in Form 4 in Chemistry Most Tested Areas in Form Four in Chemistry Most Tested Areas in Form One in Chemistry Most Tested Areas in Form Three in Chemistry Most Tested Areas in Form Two in Chemistry Most Tested Areas in Kcse Chemistry Most Tested Areas in Kcse Chemistry Exams Most Tested Areas in Senior 1 in Chemistry Most Tested Areas in Senior 2 in Chemistry Most Tested Areas in Senior 3 in Chemistry Most Tested Areas in Senior 4 in Chemistry Most Tested Areas in Senior Four in Chemistry Most Tested Areas in Senior One in Chemistry Most Tested Areas in Senior Three in Chemistry Most Tested Areas in Senior Two in Chemistry Most Tested Areas in Uneb Chemistry Revision Notes Chemistry Form 1 - Free Kcse Past Papers Who Was Chemistry Champion KCSE 2019 Top 100 Students in Chemistry KCSE 2019 Best 100 Students in Chemistry KCSE 2019 Top Student in Chemistry KCSE Best Student in Chemistry KCSE Who Was Chemistry Champion KCSE 2020 Top 100 Students in Chemistry KCSE 2020 Best 100 Students in Chemistry KCSE 2020 Names of Best Chemistry Students KCSE Names of Top Chemistry Students KCSE High School Chemistry Notes Pdf Best High School Chemistry Notes Pdf Comprehensive High School Chemistry Notes Pdf Klb Chemistry Form 1 Book Pdf Chemistry Form 1 Best Notes Chemistry Form 1 Notes Pdf Chemistry Form 1 Pressure Chemistry Form 1 Questions and Answers Chemistry Form 1 Questions and Answers Pdf Download Chemistry Form 2 Best Notes Chemistry Form 3 Best Notes Chemistry Form 4 Best Notes Chemistry Form Four Best Notes Chemistry Form One Best Notes Chemistry Form Three Best Notes Chemistry Form Two Best Notes Chemistry Notes Form 1 to 4 Chemistry Notes Pdf
Scholarship 2025/26
Current Scholarships 2025/2026 - Fully FundedFull Undergraduate Scholarships 2025 - 2026
Fully Funded Masters Scholarships 2025 - 26
PhD Scholarships for International Students - Fully Funded!
Funding Opportunities for Journalists 2025/2026
Funding for Entrepreneurs 2025/2026
***
